Abstract
Research Article
Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformants
Shu Wei*, Muhammad Abdullah, Ferdinand L Shamalla and Mohammad M Rana
Published: 12 June, 2017 | Volume 1 - Issue 1 | Pages: 044-051
Agrobacterium rhizogenes ATCC 15834 wild type strain was transformed with the binary vector pBI121 using the heat shock method. The transformed Agrobacterium was then tested for virulence through tobacco leaf explant transformation. Compared to the non-transformed Agrobacterium, the transformed Agrobacterium showed reduced virulence, producing significantly lower number of hairy roots in tobacco leaf explants. Although the transformed Agrobacterium showed reduced virulence, it was able to transfer the T-DNA of the binary vector into the plant genome, resulting in stable GUS expression in the generated hairy roots. This indicated that in addition to the transfer DNA (T-DNA) from its root inducing (Ri) plasmid, the transformed Agrobacterium is also capable of transferring the binary vector T-DNA and allowing the integration of a foreign gene. Results also showed that hairy root generation efficiency of the transformed Agrobacterium varied with the concentration of the selection agent (kanamycin). Hairy root generation efficiency (hairy roots·explant-1) progressively increased with decreasing concentrations of kanamycin; and the efficiency was highest in the absence of kanamycin. Generated hairy roots showed very strong to tiny GUS expression even those that grew under the highest concentration of the kanamycin (50 mg·L-1). This indicated that co-transformation and efficient transgene expression does not always occur.
Read Full Article HTML DOI: 10.29328/journal.jpsp.1001005 Cite this Article Read Full Article PDF
Keywords:
A. Rhizogenes; Hairy root; Virulence; GUS
References
- Dunwell JM. Transgenic approaches to crop improvement. J Exp Bot. 2000; 51: 487-496. Ref.: https://goo.gl/RMeC4x
- Ingelbrecht I, Breyne P, Vancompernolle K, Jacobs A, Van Montagu M, et al. Transcriptional interferences in transgenic plants. Gene. 1991; 109: 239-242. Ref.: https://goo.gl/4GSpst
- Conn HJ. Validity of the genus Alcaligenes. J Bacteriol. 1942; 44: 353-360. Ref.: https://goo.gl/vYEOUl
- Veena V, Taylor CG. Agrobacterium rhizogenes: recent developments and promising applications. In vitro Cell. 2007; 43: 383-403. Ref.: https://goo.gl/0Yuga3
- Gelvin SB. Improving plant genetic engineering by manipulating the host. Trends Biotechnol. 2003; 21: 95-98. Ref.: https://goo.gl/pk440K
- Chandra S. Natural plant genetic engineer Agrobacterium rhizogenes: role of T-DNA in plant secondary metabolism. Biotechnol Lett. 2012; 34: 407-415. Ref.: https://goo.gl/xewU9B
- Chilton MD, Tepfer DA, Petit A, David C, Casse-Delbart F, et al. Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature. 1982; 295: 432-434. Ref.: https://goo.gl/MWWaeV
- Lee S, Blackhall NW, Power JB, Cocking EC, Tepfer D, et al. Genetic and morphological transformation of rice with the rolA gene from the Ri TL-DNA of Agrobacterium rhizogenes. Plant Sci. 2001; 161: 917-925. Ref.: https://goo.gl/g8NPV4
- Rao SR, Ravishankar G. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002; 20: 101-153. Ref.: https://goo.gl/8GHsi4
- Shanks JV, Morgan J. Plant ‘hairy root’ culture. Curr Opin Biotech. 1999; 10: 151-155. Ref.: https://goo.gl/t787o4
- Kim YJ, Wyslouzil BE, Weathers PJ. Secondary metabolism of hairy root cultures in bioreactors. In vitro Cell. 2002; 38: 1-10. Ref.: https://goo.gl/6wQP7d
- Nader BL, Taketa AT, Pereda-Miranda R, Villarreal ML. Production of triterpenoids in liquid-cultivated hairy roots of Galphimia glauca. Planta Med. 2006; 72: 842-844. Ref.: https://goo.gl/5b4uVk
- Bensaddek L, Villarreal ML, Fliniaux MA. Induction and growth of hairy roots for the production of medicinal compounds. Electron J Integr Biosci. 2008; 3: 2-9. Ref.: https://goo.gl/mtI0Jv
- Hamill JD, Prescott A, Martin C. Assessment of the efficiency of cotransformation of the T-DNA of disarmed binary vectors derived from Agrobacterium tumefaciens and the T-DNA of A. rhizogenes. Plant Mol Biol. 1987; 9: 573-584. Ref.: https://goo.gl/wSN8Cp
- Limpens E, Ramos J, Franken C, Raz V, Compaan B, et al. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J Exp Bot. 2004; 55: 983-992. Ref.: https://goo.gl/lorYQ3
- Preiszner J, van Toai TT, Huynh L, Bolla RI, Yen HH. Structure and activity of a soybean Adh promoter in transgenic hairy roots. Plant Cell Rep. 2001; 20: 763-769. Ref.: https://goo.gl/I9qGRm
- Menzel G, Harloff HJ, Jung C. Expression of bacterial poly (3-hydroxybutyrate) synthesis genes in hairy roots of sugar beet (Beta vulgaris L.). Appl Microbiol Biot. 2003; 60: 571-576. Ref.: https://goo.gl/LGVWec
- Sharp JM, Doran PM. Strategies for enhancing monoclonal antibody accumulation in plant cell and organ cultures. Biotechnol Progr. 2001; 17: 979-992. Ref.: https://goo.gl/lrFcdz
- Mitra A, Mayer MJ, Mellon FA, Michael AJ, Narbad A, et al. 4-hydroxycinnamoyl-CoA hydratase/lyase, an enzyme of phenylpropanoid cleavage from Pseudomonas, causes formation of C6–C1 acid and alcohol glucose conjugate when expressed in hairy root of Datura stramonium L. Planta. 2002; 215: 79-89. Ref.: https://goo.gl/1VGMmL
- Seki H, Ohyama K, Nishizawa T, Yoshida S, Muranaka T. The ‘‘all-in-one’’ rol-type binary vectors as a tool for functional genomics studies using hairy roots. Plant Biotechnol. 2008; 25: 347-355. Ref.: https://goo.gl/O0LBX2
- Hansen J, Jurgensen JE, Stougaard J, Marcker KA. Hairy roots–a short cut to transgenic root nodules. Plant Cell Rep. 1989; 8: 12-15. Ref.: https://goo.gl/b2g1Io
- Ohara A, Akasaka Y, Daimon H, Mii M. Plant regeneration from hairy roots induced by infection with Agrobacterium rhizogenes in Crotalaria juncea L. Plant Cell Rep. 2000; 19: 563-568. Ref.: https://goo.gl/OXIdKC
- Crane C, Wright E, Dixon RA, Wang ZY. Transgenic Medicago truncatula plants obtained from Agrobacterium tumefaciens-transformed roots and Agrobacterium rhizogenes-transformed hairy roots. Planta. 2006; 223: 1344-1354. Ref.: https://goo.gl/1jgvba
- Vinterhalter B, Savic J, Platis AJ, Raspor M, Ninkovic S, et al. Nickel tolerance and hyperaccumulation in shoot cultures regenerated from hairy root cultures of Alyssum murale Waldst et Kit. Plant Cell Tiss Org. 2008; 94: 299-303. Ref.: https://goo.gl/V29PAh
- Gangopadhyay M, Chakraborty D, Bhattacharyya S, Bhattacharya S. Regeneration of transformed plants from hairy roots of Plumbago indica. Plant Cell Tiss Org. 2010; 102: 109-114. Ref.: https://goo.gl/rUDdH7
- Hatamoto H, Boulter ME, Shirsat AH, Croy EJ, Ellis JR. Recovery of morphologically normal transgenic tobacco from hairy roots co-transformed with Agrobacterium rhizogenes and a binary vector plasmid. Plant Cell Rep. 1990; 9: 88-92. Ref.: https://goo.gl/db0C4H
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987; 6: 3901-3907. Ref.: https://goo.gl/k2ea7Q
- Russell OF. MSTAT-C v. 2.1 (A Computer Based Data Analysis Software); Crop and Soil Science Department, Michigan State University: East Lansing, MI, USA. 1994.
- Collier R, Fuchs B, Walter N, Kevin LW, Taylor CG. Ex vitro composite plants: an inexpensive, rapid method for root biology. Plant J. 2005; 43: 449-457. Ref.: https://goo.gl/VtIlx7
- Kakkar A, Verma VK. Agrobacterium mediated biotransformation. J Appl Pharmaceut Sci. 2011; 01: 29-35. Ref.: https://goo.gl/ae6Vi9
Figures:
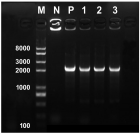
Figure 1
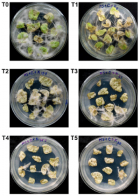
Figure 2
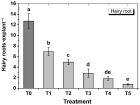
Figure 3

Figure 4
Similar Articles
-
Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformantsShu Wei*,Muhammad Abdullah,Ferdinand L Shamalla,Mohammad M Rana. Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformants. . 2017 doi: 10.29328/journal.jpsp.1001005; 1: 044-051
-
Alternative method for the transformation of Capsicum speciesZoltan Toth*,Mate Toth,Zoltan Szabo. Alternative method for the transformation of Capsicum species. . 2021 doi: 10.29328/journal.jpsp.1001053; 5: 001-003
-
Characterization and virulence determination of Colletotrichum kahawae isolates from Gidami, Western EthiopiaZenebe W*,Daniel T,Weyessa G. Characterization and virulence determination of Colletotrichum kahawae isolates from Gidami, Western Ethiopia. . 2021 doi: 10.29328/journal.jpsp.1001054; 5: 004-013
-
UPLC-Q-TOF-MS-based untargeted studies of the secondary metabolites secreted by Sclerotinia sclerotiorum under the axenic conditionNavin Chandra Gupta*,Shaweta Arora#,Aditi Kundu,Pankaj Sharma,Mahesh Rao,Ramcharan Bhattacharya. UPLC-Q-TOF-MS-based untargeted studies of the secondary metabolites secreted by Sclerotinia sclerotiorum under the axenic condition. . 2022 doi: 10.29328/journal.jpsp.1001095; 6: 173-182
Recently Viewed
-
Overview on liquid chromatography and its greener chemistry applicationAdel E Ibrahim,Magda Elhenawee,Hanaa Saleh,Mahmoud M Sebaiy*. Overview on liquid chromatography and its greener chemistry application. Ann Adv Chem. 2021: doi: 10.29328/journal.aac.1001023; 5: 004-012
-
COPD and low plasma vitamin D levels: Correlation or causality?Luca Gallelli*, Erika Cione,Stefania Zampogna, Gino Scalone. COPD and low plasma vitamin D levels: Correlation or causality? . J Pulmonol Respir Res. 2018: doi: 10.29328/journal.jprr.1001008; 2: 011-012
-
Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A ReviewDivya Khorwal*, GK Mathur, Umema Ahmed, SS Daga. Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A Review. J Forensic Sci Res. 2024: doi: 10.29328/journal.jfsr.1001057; 8: 009-015
-
Markov Chains of Molecular Processes of Biochemical MaterialsOrchidea Maria Lecian*. Markov Chains of Molecular Processes of Biochemical Materials. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001076; 7: 001-005
-
Generation of Curved Spacetime in Quantum FieldSarfraj Khan*. Generation of Curved Spacetime in Quantum Field. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001077; 7: 006-009
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."