Abstract
Commentary
Antiviral RNAi mediated Plant defense versus its suppression by viruses
Sunil Kumar Mukherjee* and Dinesh Gupta
Published: 25 January, 2019 | Volume 3 - Issue 1 | Pages: 001-008
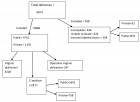
The age-old battle between plants and viruses has many twists and turns. Plants acquired the RNAi factors to checkmate the viruses and the viruses encode VSRs to defeat RNAi for their own survival. Plants designed mechanisms to neutralize the toxic effects of VSRs and the viruses, in their turn, use host microRNAs to strengthen their infection processes. The infightings between these two entities will take different shapes with prolonged evolution and accordingly the researchers will dig these novel forms of duels not only to throw lights in the involved mechanisms but also to manipulate various antiviral strategies. Some of the research courses that might come up in the immediate future are discussed.
Read Full Article HTML DOI: 10.29328/journal.jpsp.1001025 Cite this Article Read Full Article PDF
References
- Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, et al. RNA interference: biology: mechanism: and applications. Micro Mol Biol Rev. 2003; 67: 657-685. Ref.: https://goo.gl/yZVjzk
- Koonin EV, Dolja VV, Krupovic M. Origins and evolution of viruses of eukaryotes: The ultimate modularity. Virol. 2015; 479-480: 2-25. Ref.: https://goo.gl/wsJprG
- Ma A, Mondragón RJ. Rich-Cores in Networks. PLoS One. 2015; 10: 1-13. Ref.: https://goo.gl/5iN9iU
- Rajewsky, Nikolaus, Jurga, Stefan, Barciszewski. In Plant Epigenetics. RNA Tech. 199-230. Ref.: https://goo.gl/twwyMH
- Yang Z, Li Y. Dissection of RNAi-based antiviral immunity in plants. Curr Opin Virol.2018; 32: 88-99. Ref.: https://goo.gl/eNnTC8
- Llave C. Virus-derived small interfering RNAs at the core of plant-virus interactions.Trends Plant Sci. 2010; 15: 701-707. Ref.: https://goo.gl/HbDbgP
- Wang XB, Jovel J, Udomporn P, Wang Y, Wu Q, et al. The 21-Nucleotide, but Not 22-Nucleotide, Viral Secondary Small Interfering RNAs Direct Potent Antiviral Defense by Two Cooperative Argonautes in Arabidopsis thaliana [W][OA]. Plant Cell. 2011; 23: 1625-1638. Ref.: https://goo.gl/qFWGKq
- Brosseau C, El Oirdi M, Adurogbangba A, Ma X, Moffett P. Antiviral Defense Involves AGO4 in an Arabidopsis–Potexvirus Interaction. Mol Plant-Microbe Int. 2016; 29: 878-888. Ref.: https://goo.gl/vyJsNT
- Hort Res. 2018; 5: 62-75. Ref.:
- Mirian SJ, Mohammadi AR, Karimi G, Nia KI, Motamedi G, et al. Survey on helminthic and protozoan contaminations in alimentary canal of ostrich at Tehran Province slaughterhouses. Viruses. 9: 256-276. Ref.: https://goo.gl/fi3gct
- Taochy C, Gursanscky NR, Cao J, Fletcher SJ, Dressel U, et al. A Genetic Screen for Impaired Systemic RNAi Highlights the Crucial Role of DICER-LIKE 2. Plant Physiol. 2017; 175: 1424-1437. Ref.: https://goo.gl/6dd2ou
- Qin C, Li B, Fan Y, Zhang X, Yu Z, et al. Roles of Dicer-Like Proteins 2 and 4 in Intra- and Intercellular Antiviral Silencing. Plant Physiol. 2017; 174: 1067-1081. Ref.: https://goo.gl/fXhm5m
- Guo Z, Lu J, Wang X, Zhan B, Li W, et al. Lipid flippases promote antiviral silencing and the biogenesis of viral and host siRNAs in Arabidopsis. Proc Acad Natl Sci (USA). 2017; 114: 1377-1382. Ref.: https://goo.gl/VzaYr8
- Guo Z, Wang XB, Wang Y, Li WX, Gal-On A, et al. Identification of a New Host Factor Required for Antiviral RNAi and Amplification of Viral siRNAs. Plant Physiol. 2017; 176: 1587-1597. Ref.: https://goo.gl/qv7qfL
- Simón-Mateo C, García JA. MicroRNA-guided processing impairs Plum pox virus replication, but the virus readily evolves to escape this silencing mechanism. J Virol. 2006; 80: 2429-2436. Ref.: https://goo.gl/TLb9on
- Deng P, Muhammad S, Cao M, Wu L. Biogenesis and regulatory hierarchy of phased small interfering RNAs in plants. Plant Biotech J. 2018; 16: 965-975. Ref.: https://goo.gl/6y4BqU
- Bivalkar-Mehla S, Vakharia J, Mehla R, Abreha M, Kanwar JR, et al. Viral RNA silencing suppressors (RSS): novel strategy of viruses to ablate the host RNA interference (RNAi) defense system. Virus Res. 2011; 155: 1-9. Ref.: https://goo.gl/ovBczc
- Zhao JH, Hua CL, Fang YY, Guo HS. The dual edge of RNA silencing suppressors in the virus-host interactions. Curr Opin Virol. 2016; 17: 39-44. Ref.: https://goo.gl/QkS6Hz
- Csorba T, Kontra L, Burgyán J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virol. 2015; 479-480, 85-103. Ref.: https://goo.gl/mNuQoY
- Pérez-Cañamás M, Hernández C. Key importance of small RNA binding for the activity of a glycine-tryptophan (GW) motif-containing viral suppressor of RNA silencing. J Biol Chem. 2015; 290: 3106-3120. Ref.: https://goo.gl/Ankwiw
- Pertermann R, Tamilarasan S, Gursinsky T, Gambino G, Schuck J, et al. A Viral Suppressor Modulates the Plant Immune Response Early in Infection by Regulating MicroRNA Activity. mBio. 2018’ 9: e00419-18. Ref.: https://goo.gl/SL7tWw
- Incarbone M, Zimmermann A, Hammann P, Erhardt M, Michel F, et al. Neutralization of mobile antiviral small RNA through peroxisomal import. Nat Plant. 2017; 3: 17094. Ref.: https://goo.gl/oL3w2K
- Li F, Wang A. RNA decay is an antiviral defense in plants that is counteracted by viral RNA silencing suppressors. PLos Pathog. 2018; 14: e1007228. Ref.: https://goo.gl/anRxTq
- Pumplin N, Voinnet O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat Rev (Microbiol). 2013; 11: 745-760. Ref.: https://goo.gl/6yYLYd
- Chen L, Yan Z, Xia Z, Cheng Y, Jiao Z, et al. A Violaxanthin Deepoxidase Interacts with a Viral Suppressor of RNA Silencing to Inhibit Virus Amplification. Plant Physiol. 2017; 175:1774-1794. Ref.: https://goo.gl/sedWUc
- Shen Q, Hu T, Bao M, Cao L, Zhang H, et al. Tobacco RING E3 Ligase NtRFP1 Mediates Ubiquitination and Proteasomal Degradation of a Geminivirus-Encoded βC1. Mo Plant. 2016; 9: 911-925. Ref.: https://goo.gl/4HSfhS
- Jeon EJ, Tadamura K, Murakami T, Inaba JI, Kim BM, et al. rgs-CaM Detects and Counteracts Viral RNA Silencing Suppressors in Plant Immune Priming. J Virol. 2017; 91-e00761-17. Ref.: https://goo.gl/D5UYNM
- Várallyay E, Válóczi A, Agyi A, Burgyán J, Havelda Z. Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. EMBO J. 2010; 29: 3507-3519. Ref.: https://goo.gl/LXHHCa
- Zheng L, Zhang C, Shi C, Yang Z, Wang Y, et al. Rice stripe virus NS3 protein regulates primary miRNA processing through association with the miRNA biogenesis factor OsDRB1 and facilitates virus infection in rice. PLos Pathog. 2017; 13: e1006662. Ref.: https://goo.gl/uxY2XZ
- Scientific Repts. 2016; 6: 20167-20180.
- Feng J, Liu S, Wang M, Lang Q, Jin C. Identification of microRNAs and their targets in tomato infected with Cucumber mosaic virus based on deep sequencing. Planta. 2014; 240: 1335-1352. Ref.: https://goo.gl/nLh15N
- Iki T, Cléry A, Bologna NG, Sarazin A, Brosnan CA, et al. Structural Flexibility Enables Alternative Maturation, ARGONAUTE Sorting and Activities of miR168, a Global Gene Silencing Regulator in Plants. Mol Plant. 2018; 11: 1008-1023. Ref.: https://goo.gl/xknqL2
- Várallyay E, Havelda Z. Unrelated viral suppressors of RNA silencing mediate the control of ARGONAUTE1 level. Mol Plant Pathol. 2013; 14: 567-575. Ref.: https://goo.gl/aqxqUd
- Noman A, Aqeel M, Deng J, Khalid N, Sanaullah T, et al. Biotechnological Advancements for Improving Floral Attributes in Ornamental Plants. Front Plant Sci. 2017; 8: 1760-1769. Ref.: https://goo.gl/x7nhm6
- Honda T, Yamamoto K, Yo A, Takarada Y, Shibata H. PCR primer to detect cholera toxin-producing Vibrio cholera. ELIFE: Microbiol and Infect. diseses/ Plant Biol. 2015; 4: Ref.: https://goo.gl/8jH14n
- Kravchik M, Sunkar R, Damodharan S, Stav R, Zohar M, et al. Global and local perturbation of the tomato microRNA pathway by a trans-activated DICER-LIKE 1 mutant. J Exp Bot. 2014; 65: 725-739. Ref.: https://goo.gl/xcb58y
- Wang Z, Hardcastle TJ, Canto Pastor A, Yip WH, Tang S, et al. A novel DCL2-dependent miRNA pathway in tomato affects susceptibility to RNA viruses. Genes and Dev. 2018; 32: 1155-1160. Ref.: https://goo.gl/Jrii6P
- Virology J. 2010; 7: 281-296.
- Zhang C, Ding Z, Wu K, Yang L, Li Y, et al. Suppression of Jasmonic Acid-Mediated Defense by Viral-Inducible MicroRNA319 Facilitates Virus Infection in Rice. Mol Plant. 2016; 9: 1302-1314. Ref.: https://goo.gl/TCkmMe
- Cillo F, Mascia T, Pasciuto MM, Gallitelli D. Differential effects of mild and severe Cucumber mosaic virus strains in the perturbation of MicroRNA-regulated gene expression in tomato map to the 3' sequence of RNA 2. Mol Plant-Microbe Int. 2009; 22: 1239-1249. Ref.: https://goo.gl/bgTdAi
- Obbard DJ, Gordon KH, Buck AH, Jiggins FM. The evolution of RNAi as a defence against viruses and transposable elements. Phil Trans R Soc B. 2009; 364: 99-115. Ref.: https://goo.gl/3CEeFo
- Aguado LC, Schmid S, May J, Sabin LR, Panis M, et al. RNase III nucleases from diverse kingdoms serve as antiviral effectors. Nature. 2017; 547: 114-117. Ref.: https://goo.gl/HoRmYN
- Vazquez F, Blevins T, Ailhas J, Boller T, Meins F, Jr. Evolution of Arabidopsis MIR genes generates novel microRNA classes. Nucl Ac Res. 2008; 36: 6429-6438. Ref.: https://goo.gl/bWbgvQ
- Ying XB, Dong L, Zhu H, Duan CG, Du QS, et al. RNA-Dependent RNA Polymerase 1 from Nicotiana tabacum Suppresses RNA Silencing and Enhances Viral Infection in Nicotiana benthamiana. Plant Cell. 2010; 22: 1358-1372. Ref.: https://goo.gl/snkReA
- Ishibashi K, Kezuka Y, Kobayashi C, Kato M, Inoue T, et al. Structural basis for the recognition–evasion arms race between Tomato mosaic virus and the resistance gene Tm-1. Proc Natl Acd Sc. U S A. 2014; 111: E3486-E3495. Ref.: https://goo.gl/vjZTfo
Similar Articles
-
Impact of Calcium Phosphate Nanoparticles on Rice PlantHrishikesh Upadhyaya*,Lutfa Begum,Bishal Dey,P K Nath,S K Panda. Impact of Calcium Phosphate Nanoparticles on Rice Plant. . 2017 doi: 10.29328/journal.jpsp.1001001; 1: 001-010
-
The Effects of Pharmacological Carbonic Anhydrase Suppression on Defence Responses of Potato Leaves To Phytophthora InfestansMagdalena Arasimowicz-Jelonek*,Jolanta Floryszak-Wieczorek. The Effects of Pharmacological Carbonic Anhydrase Suppression on Defence Responses of Potato Leaves To Phytophthora Infestans. . 2017 doi: 10.29328/journal.jpsp.1001002; 1: 011-025
-
Phytochemical content of leaf and stem of Marsilea quadrifolia (L.)Rajangam Udayakumar*,Karikalan Gopalakrishnan. Phytochemical content of leaf and stem of Marsilea quadrifolia (L.). . 2017 doi: 10.29328/journal.jpsp.1001003; 1: 026-037
-
Antagonistic features displayed by Plant Growth Promoting Rhizobacteria (PGPR): A ReviewMohsin Tariq*,Muhammad Noman,Temoor Ahmed,Amir Hameed,Natasha Manzoor,Marriam Zafar. Antagonistic features displayed by Plant Growth Promoting Rhizobacteria (PGPR): A Review . . 2017 doi: 10.29328/journal.jpsp.1001004; 1: 038-043
-
Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformantsShu Wei*,Muhammad Abdullah,Ferdinand L Shamalla,Mohammad M Rana. Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformants. . 2017 doi: 10.29328/journal.jpsp.1001005; 1: 044-051
-
Effects of Vochysia haenkeana extract on the neuromuscular blockade induced by Bothrops jararaca venom on chick biventer cervicis preparation in vitroYoko Oshima-Franco*,Fernanda Dias da Silva,Natália Tribuiani,Isadora Caruso Fontana Oliveira,Regina Yuri Hashimoto Miura,Rafael S Floriano,Márcio Galdino dos Santos,Sandro Rostelato-Ferreira. Effects of Vochysia haenkeana extract on the neuromuscular blockade induced by Bothrops jararaca venom on chick biventer cervicis preparation in vitro. . 2017 doi: 10.29328/journal.jpsp.1001006; 1: 052-058
-
HBV: Genomic Structure, HBVsAg Isolation and innovative Virotherapy Initiation in the Middle EastAboul-Ata E Aboul-Ata*,Essam M Janahi,I M El-Kalamawy,Kathleen Hefferon,Amal Mahmoud. HBV: Genomic Structure, HBVsAg Isolation and innovative Virotherapy Initiation in the Middle East . . 2017 doi: 10.29328/journal.jpsp.1001007; 1: 059-061
-
Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seedUpadhyaya H*,Roy H,Shome S,Tewari S,Bhattacharya MK,Panda SK. Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seed . . 2017 doi: 10.29328/journal.jpsp.1001008; 1: 062-070
-
Effects of Site Factors on the Clonal Growth of Phyllostachys bambusoides f. shouzhu YiXiaohong Gan*,Lijuan Chen,Cuibin Tang,Xia Zhang. Effects of Site Factors on the Clonal Growth of Phyllostachys bambusoides f. shouzhu Yi. . 2017 doi: 10.29328/journal.jpsp.1001009; 1: 071-079
-
Evaluation of genetic diversity in germplasm of paprika (Capsicum spp.) using random amplified polymorphic DNA (RAPD) markersRueda-Puente EO*,Renganathan P,Ruíz-Alvarado C,Hernández-Montiel LG,Prasath Duraisamy. Evaluation of genetic diversity in germplasm of paprika (Capsicum spp.) using random amplified polymorphic DNA (RAPD) markers. . 2017 doi: 10.29328/journal.jpsp.1001010; 1: 080-086
Recently Viewed
-
Synthesis of Carbon Nano Fiber from Organic Waste and Activation of its Surface AreaHimanshu Narayan*,Brijesh Gaud,Amrita Singh,Sandesh Jaybhaye. Synthesis of Carbon Nano Fiber from Organic Waste and Activation of its Surface Area. Int J Phys Res Appl. 2019: doi: 10.29328/journal.ijpra.1001017; 2: 056-059
-
Obesity Surgery in SpainAniceto Baltasar*. Obesity Surgery in Spain. New Insights Obes Gene Beyond. 2020: doi: 10.29328/journal.niogb.1001013; 4: 013-021
-
Tamsulosin and Dementia in old age: Is there any relationship?Irami Araújo-Filho*,Rebecca Renata Lapenda do Monte,Karina de Andrade Vidal Costa,Amália Cinthia Meneses Rêgo. Tamsulosin and Dementia in old age: Is there any relationship?. J Neurosci Neurol Disord. 2019: doi: 10.29328/journal.jnnd.1001025; 3: 145-147
-
Case Report: Intussusception in an Infant with Respiratory Syncytial Virus (RSV) Infection and Post-Operative Wound DehiscenceLamin Makalo*,Orlianys Ruiz Perez,Benjamin Martin,Cherno S Jallow,Momodou Lamin Jobarteh,Alagie Baldeh,Abdul Malik Fye,Fatoumatta Jitteh,Isatou Bah. Case Report: Intussusception in an Infant with Respiratory Syncytial Virus (RSV) Infection and Post-Operative Wound Dehiscence. J Community Med Health Solut. 2025: doi: 10.29328/journal.jcmhs.1001051; 6: 001-004
-
The prevalence and risk factors of chronic kidney disease among type 2 diabetes mellitus follow-up patients at Debre Berhan Referral Hospital, Central EthiopiaGetaneh Baye Mulu,Worku Misganew Kebede,Fetene Nigussie Tarekegn,Abayneh Shewangzaw Engida,Migbaru Endawoke Tiruye,Mulat Mossie Menalu,Yalew Mossie,Wubshet Teshome,Bantalem Tilaye Atinafu*. The prevalence and risk factors of chronic kidney disease among type 2 diabetes mellitus follow-up patients at Debre Berhan Referral Hospital, Central Ethiopia. J Clini Nephrol. 2023: doi: 10.29328/journal.jcn.1001104; 7: 025-031
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."