Abstract
Research Article
Comprehensive phenotypic characterization and genetic distinction of distinct goosegrass (Eleusine indica L. Gaertn.) ecotypes
Robert A Kerr*, Tatyana Zhebentyayeva, Christopher Saski and Lambert B McCarty
Published: 04 October, 2019 | Volume 3 - Issue 3 | Pages: 095-100
Goosegrass (Eleusine indica L. Gaertn.) is a troublesome weed in turfgrass systems throughout the world. The development of herbicide resistant ecotypes has occurred to multiple modes of action. Goosegrass is a prolific seed producer (~50,000 per plant), fast growing and diverse weed. Such growing attributes make it essential to have a better understanding of the genetic diversity of various ecotypes. The objectives of this study were to determine if morphologically distinct goosegrass ecotypes collected in Florida were phenotypically distinct and genetically different. Phenotypically, the goosegrass ecotypes can be classified as follows; dwarf, intermediate 1 (int_I), intermediate 2 (int_II) and wild. The dwarf had the least seedheads followed by the wild ecotype; 5 and 17 respectively, while int_I and int_II had highest number of seedheads; 22 and 34 respectively. The dwarf ecotype had lowest height of 6 cm and the wild ecotype had highest height of 36 cm. Dwarf and int_II ecotypes had shortest internode length of 0.2 cm and 1 cm, respectively, while the wild ecotype had longest internode length of 7 cm. The dwarf ecotype had lowest number of racemes per plant of 1, while the wild ecotype had highest number of racemes per plant of 7. Total biomass was lowest for the dwarf and int_II ecotype; 0.7 g and 1.5 g, respectively, and total biomass was highest for the wild ecotype at 5 g. Gene sequencing of two rice (Oryza) gene sequences (accession AP014964 (gene A) and AP014965 (gene B)) and subsequent phylogenetic analysis suggest the ecotypes are genetically different. Three single nucleotide polymorphisms (SNP) of interest were discovered indicating allelic differences between ecotypes.
Read Full Article HTML DOI: 10.29328/journal.jpsp.1001038 Cite this Article Read Full Article PDF
Keywords:
Eleusine indica L. Gaertn.; Turfgrass; Weed control
References
- Agrawal AA. Phenotypic plasticity in the interactions and evolution of species. Science. 2001; 294: 321-326. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11598291
- Sultan SE. Phenotypic plasticity and plant adaptation. Acta Bot Neerl. 1995; 44: 363-383.
- Geng YP, Pan XY, Xu CY, Zhang WJ, Li B, et al. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligatorweed to colonize a wide range of habitats. Biological Invasions. 2007; 9: 245-256.
- Saidi N, Kadir J, Hong LW. Genetic diversity and morphological variations of goosegrass [Eleusine indica (L.) Gaertn] ecotypes in Malaysia. Weed & Turfgrass Sci. 2016; 5: 144-154.
- Chen J, Huang Z, Huang H, Wei S, Liu Y, et al. Selection of relatively exact reference genes for gene expression studies in goosegrass (Eleusine indica) under herbicide stress. Scientific Reports. 2017; 7: 46494.
- Ganeshaiah KN, Shaanker RU. Evolution of reproductive behavior in the genus Eleusine. Euphytica. 1982; 31: 397-404.
- Brosnan JT, Breeden GK. Herbicide resistance in turfgrass: an emerging problem? Outlooks on Pest Manag. 2013; 24: 164-168.
- Lee LJ, Ngim J. A first report of glyphosate?resistant goosegrass (Eleusine indica (L.) Gaertn) in Malaysia. Pest Manag Sci. 2000; 56: 336-339.
- Buker RS, Steed ST, Stall WM. Confirmation and control of a paraquat-tolerant goosegrass (Eleusine indica) biotype. Weed Technol. 2002; 16: 309-313.
- Brosnan JT, Nishimoto RK, de Frank J. Metribuzin-resistant goosegrass (Eleusine indica) in bermudagrass turf. Weed Technol. 2008; 22: 675-678.
- Seng CT, Van Lun L, San CT, Sahid IB. Initial report of glufosinate and paraquat multiple resistance that evolved in a biotype of goosegrass (Eleusine indica) in Malaysia. Weed Biol and Manag. 2010; 10: 229-233.
- McElroy JS, Head WB, Wehtje GR, Spak D. Identification of goosegrass (Eleusine indica) biotypes resistant to preemergence-applied oxadiazon. Weed Technol. 2017; 31: 675-681
- Busey P. Goosegrass (Eleusine indica) control with foramsulfuron in bermudagrass (Cynodon spp.) turf. Weed Technol. 2004; 18: 634-640.
- McCarty LB. Goosegrass (Eleusine indica) control in bermudagrass (Cynodon spp.) turf by diclofop. Weed Sci. 1991; 39: 255-261.
- Burdon JJ. Diseases and Plant Population Biology. Cambridge University Press, Cambridge, UK, 1987.
- Cross RB, McCarty LB, McElroy JS, Tharayil N, Bridges WC. Comparison of enzyme and growth characteristics in ALS-inhibitor susceptible and resistant annual bluegrass (Poa annua) biotypes. Weed Sci. 2015; 63: 220-228.
- Dekker J. Weed diversity and weed management. Weed Sci. 1997; 357-363.
- Varma V, Osuri AM. Black spot: A platform for automated and rapid estimation of leaf area from scanned images. Plant Ecol. 2013; 214: 1529-1534.
- Kubisiak TL, Nelson CD, Staton ME, Zhebentyayeva T, Smith C, et al. A transcriptome-based genetic map of Chinese chestnut (Castanea mollissima) and identification of regions of segmental homology with peach (Prunus persica). Tree Genetics & Genomes. 2013; 9: 557-571.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215: 403-410. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2231712
- Kanapeckas KL, Vigueira CC, Ortiz A, Gettler KA, Burgos NR, et al. Escape to ferality: the endoferal origin of weedy rice from crop rice through de-domestication. PLoS One. 2016; 11: e0162676. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035073/
- Chen S, McElroy JS, Dane F, Peatman E. Optimizing transcriptome assemblies for Eleusine indica leaf and seedling by combining multiple assemblies from three de novo assemblers. Plant Genome. 2015; 8: 1-10.
- Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2?macroglobulin and tau: a population? based autopsy study. Ann Med. 2008; 40: 232-239. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18382889
- Garcia NS, Sexton J, Riggins T, Brown J, Lomas MW, et al. High variability in cellular stoichiometry of carbon, nitrogen, and phosphorus within classes of marine eukaryotic phytoplankton under sufficient nutrient conditions. Frontiers in Microbiology. 2018; 27: 543. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5880891/
- Wilkins O, Hafemiester C, Plessis A, Holloway-Phillips MM, Pham GM, et al. Environmental gene regulatory influence networks in rice (Oryza sativa): response to water deficit, high temperature and agricultural environments. BioRxiv. 2016; 1: 042317.
- Chandran AK, Jeong HY, Jung KH, Lee C. Development of functional modules based on co-expression patterns for cell-wall biosynthesis related genes in rice. J Plant Biol. 2016; 59: 1-5.
- Lund B. Repatriation of Nordic barley germplasm. Ph.D Dissertation. Copenhagen, Denmark: The Royal Veterinary and Agricultural University. 2002.
- Li F, Gan S, Weng Q, Zhao X, Huang S, et al. RAPD and morphological diversity among four populations of the tropical tree species Paramichelia baillonii (Pierre) Hu in China. Forest Ecology and Manag. 2008; 255: 1793-801.
- Vetelainen M, Gammelgard E, Valkonen JP. Diversity of Nordic landrace potatoes (Solanum tuberosum L.) revealed by AFLPs and morphological characters. Genetic Resources and Crop Evolution. 2005; 52: 999-1010.
Figures:

Figure 1
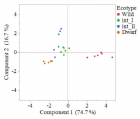
Figure 2

Figure 3
Similar Articles
-
Comprehensive phenotypic characterization and genetic distinction of distinct goosegrass (Eleusine indica L. Gaertn.) ecotypesRobert A Kerr*,Tatyana Zhebentyayeva,Christopher Saski,Lambert B McCarty. Comprehensive phenotypic characterization and genetic distinction of distinct goosegrass (Eleusine indica L. Gaertn.) ecotypes. . 2019 doi: 10.29328/journal.jpsp.1001038; 3: 095-100
-
In vitro and preventative field evaluations of potential biological control agents and synthetic fungicides for control of Clarireedia jacksonii sp. nov.Robert A Kerr*,Jeffery W Marvin,Lambert B McCarty,William C Bridges,S Bruce Martin,Christina E Wells. In vitro and preventative field evaluations of potential biological control agents and synthetic fungicides for control of Clarireedia jacksonii sp. nov.. . 2020 doi: 10.29328/journal.jpsp.1001043; 4: 001-008
-
Strobilurins: New group of fungicidesRasha E Selim*,Mohamed S Khalil. Strobilurins: New group of fungicides. . 2021 doi: 10.29328/journal.jpsp.1001062; 5: 63-064
-
Efficacy of Sequential Applications of Pendimethalin 500 EC (Pendimight®) and Oxyfluofen 240 EC (Harris®) for Weed Control in Direct Seeded Onion (Allium cepa L.) in SudanMohamed Baha Saeed*, Salah Eltom Elamin, Mark D Laing. Efficacy of Sequential Applications of Pendimethalin 500 EC (Pendimight®) and Oxyfluofen 240 EC (Harris®) for Weed Control in Direct Seeded Onion (Allium cepa L.) in Sudan. . 2024 doi: 10.29328/journal.jpsp.1001138; 8: 084-089
Recently Viewed
-
Pneumothorax as Complication of CT Guided Lung Biopsy: Frequency, Severity and Assessment of Risk FactorsGaurav Raj*,Neha Kumari,Neha Singh,Kaustubh Gupta,Anurag Gupta,Pradyuman Singh,Hemant Gupta. Pneumothorax as Complication of CT Guided Lung Biopsy: Frequency, Severity and Assessment of Risk Factors. J Radiol Oncol. 2025: doi: 10.29328/journal.jro.1001075; 9: 012-016
-
The Police Power of the National Health Surveillance Agency – ANVISADimas Augusto da Silva*,Rafaela Marinho da Silva. The Police Power of the National Health Surveillance Agency – ANVISA. Arch Cancer Sci Ther. 2024: doi: 10.29328/journal.acst.1001046; 8: 063-076
-
A Comparative Study of Serum Sodium and Potassium Levels across the Three Trimesters of PregnancyOtoikhila OC and Seriki SA*. A Comparative Study of Serum Sodium and Potassium Levels across the Three Trimesters of Pregnancy. Clin J Obstet Gynecol. 2023: doi: 10.29328/journal.cjog.1001137; 6: 108-116
-
Chaos to Cosmos: Quantum Whispers and the Cosmic GenesisOwais Farooq*,Romana Zahoor*. Chaos to Cosmos: Quantum Whispers and the Cosmic Genesis. Int J Phys Res Appl. 2025: doi: 10.29328/journal.ijpra.1001107; 8: 017-023
-
Phytochemical Compounds and the Antifungal Activity of Centaurium pulchellum Ethanol Extracts in IraqNoor Jawad Khadhum, Neepal Imtair Al-Garaawi*, Antethar Jabbar Al-Edani. Phytochemical Compounds and the Antifungal Activity of Centaurium pulchellum Ethanol Extracts in Iraq. J Plant Sci Phytopathol. 2024: doi: 10.29328/journal.jpsp.1001137; 8: 079-083
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."