Abstract
Research Article
Ion transporters and their molecular regulation mechanism in plants
Faiçal Brini* and Walid Saibi
Published: 25 May, 2021 | Volume 5 - Issue 2 | Pages: 028-043
With the global population predicted to grow by at least 25% by 2050, the need for sustainable production of nutritious foods is important for human and environmental health. Recent progress demonstrate that membrane transporters can be used to improve yields of staple crops, increase nutrient content and resistance to key stresses, including salinity, which in turn could expand available arable land. Exposure to salt stress affects plant water relations and creates ionic stress in the form of the cellular accumulation of Na+ and Cl− ions. However, salt stress also impacts heavily on the homeostasis of other ions such as Ca2+, K+, and NO3- and therefore requires insights into how transport and compartmentation of these nutrients are altered during salinity stress. Since Na+ interferes with K+ homeostasis, maintaining a balanced cytosolic Na+/K+ ratio has become a key salinity tolerance mechanism. Achieving this homeostatic balance requires the activity of Na+ and K+ transporters and/or channels. The aim of this review is to seek answers to this question by examining the role of major ions transporters and channels in ions uptake, translocation and intracellular homeostasis in plants.
Read Full Article HTML DOI: 10.29328/journal.jpsp.1001058 Cite this Article Read Full Article PDF
Keywords:
Ion Transporters; Na+ sensing; Na+ transport; Potassium; Proton Pumps; Salinity
References
- Shrivastava P, Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015; 22: 123-131. PubMed: https://pubmed.ncbi.nlm.nih.gov/25737642/
- Shahbaz M, Ashraf M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013; 32: 237–249.
- Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005; 167: 645–663. PubMed: https://pubmed.ncbi.nlm.nih.gov/16101905/
- Jamil A, Riaz S, Ashraf M, Foolad MR. Gene expression profiling of plants under salt stress. Crit Rev Plant Sci. 2011; 30: 435–458.
- van Ittersum MK, van Bussel LGJ, Wolf J, Grassini P, et al. Can sub-Saharan Africa feed itself? Proc. Natl Acad Sci. U.S.A. 2016; 113: 14964–14969. PubMed: https://pubmed.ncbi.nlm.nih.gov/27956604/
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008; 59: 651–681. PubMed: https://pubmed.ncbi.nlm.nih.gov/18444910/
- Cramer GR, Läuchli A, Polito VS. Displacement of Ca2+ by Na+ from the plasmalemma of root cells: a primary response to salt stress? Plant Physiol. 1985; 79: 207–211. PubMed: https://pubmed.ncbi.nlm.nih.gov/16664372/
- Kinraide TB. Interactions among Ca2+, Na+ and K+ in salinity toxicity: quantitative resolution of multiple toxic and ameliorative effects. J Exp Bot. 1999; 50: 1495–1505.
- Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003; 6: 441–445. PubMed: https://pubmed.ncbi.nlm.nih.gov/12972044/
- Marin K, Suzuki I, Yamaguchi K, Ribbeck K, Yamamoto H, et al. Identification of histidine kinases that act as sensors in the perception of salt stress in Synechocystis sp. PCC 6803. Proc Natl Acad Sci. U.S.A. 2003; 100: 9061–9066. PubMed: https://pubmed.ncbi.nlm.nih.gov/12853569/
- Tran LSP, Urao T, Qin F, Maruyama K, Kakimoto T, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci. U.S.A. 2007; 104: 20623–20628. PubMed: https://pubmed.ncbi.nlm.nih.gov/18077346/
- Shabala S, Wu H, Bose J. Salt stress sensing and early signaling events in plant roots: current knowledge and hypothesis. Plant Sci. 2015; 109–119. PubMed: https://pubmed.ncbi.nlm.nih.gov/26706063/
- Sun J, Zhang X, Deng S, Zhang C, Wang M, et al. Extracellular ATP signaling is mediated by H2O2 and cytosolic Ca2+ in the salt response of Populus euphratica cells. PLoS One 2012; 7: e53136.
- Wu H, Shabala L, Liu X, Azzarello E, Zhou M, et al. Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Front Plant Sci. 2015; 6: 71.
- Wu H. Tissue Specificity of Cytosolic K+ Retention, Na+ Extrusion, and Vacuolar Na+ Sequestration Traits in the Context of Differential Salinity Stress Tolerance in Barley and Wheat (Ph.D. Dissertation) University of Tasmania, Australia. 2015. PubMed: https://eprints.utas.edu.au/23053/
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Lett. 2007; 581: 2247–2254. PubMed: https://pubmed.ncbi.nlm.nih.gov/17459382/
- Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1controls long-distance Na+ transport in plants. Plant Cell 2002; 14: 465–477. PubMed: https://pubmed.ncbi.nlm.nih.gov/11884687/
- Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, et al. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009a; 151: 210–222. PubMed: https://pubmed.ncbi.nlm.nih.gov/19571313/
- Al-Karaki GN. Growth, water use efficiency, and sodium and potassium acquisition by tomato cultivars grown under salt stress. J Plant Nutr. 2000; 23: 1–8.
- Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, et al. HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol. 2007; 143: 1918–1928. PubMed: https://pubmed.ncbi.nlm.nih.gov/17322337/
- James RA, Blake C, Byrt CS, Munns R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and water-logged conditions. J Exp Bot. 2011; 62: 2939–2947. PubMed: https://pubmed.ncbi.nlm.nih.gov/21357768/
- Møller IS, Tester M. Salinity tolerance of Arabidopsis: A good model for cereals? Trends Plant Sci. 2007; 12: 534–540. PubMed: https://pubmed.ncbi.nlm.nih.gov/18023242/
- Munns R, James RA, Xu B, Athman A, Conn SJ, et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat Biotechnol. 2012; 30: 360–364. PubMed: https://pubmed.ncbi.nlm.nih.gov/22407351/
- Roy SJ, Huang W, Wang XJ, Evrard A, Schmöckel SM, et al. A novel protein kinase involved in Na+ exclusion revealed from positional cloning. Plant Cell Environ. 2013; 36: 553–568. PubMed: https://pubmed.ncbi.nlm.nih.gov/22897323/
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Sci. 1999; 285: 1256–1258. PubMed: https://pubmed.ncbi.nlm.nih.gov/10455050/
- Mansour MMF, Salama KHA, Al-Mutawa MM. Transport proteins and salt tolerance in plants. Plant Sci. 2003; 164: 891–900.
- Rahnama A, Poustini K, Tavakkol-Afshari R, Ahmadi A, Alizadeh H. Growth properties and ion distribution in different tissues of bread wheat genotypes (Triticum aestivum L.) differing in salt tolerance. J Agron Crop Sci. 2011; 197; 21–30.
- Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot. 1999; 84: 123–133.
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol. 2001; 19: 765–768. PubMed: https://pubmed.ncbi.nlm.nih.gov/11479571/
- Chen H, An R, Tang JH, Cui XH, Hao FS, et al. Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol Breed. 2007; 19: 215–225.
- Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K. Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Mol Biol. 2012; 79: 137–155. PubMed: https://pubmed.ncbi.nlm.nih.gov/22415161/
- Cuin TA, BOSE J, Stefano G, JHA D, Tester M, et al. Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant Cell Environ. 2011; 34: 947–961. PubMed: https://pubmed.ncbi.nlm.nih.gov/21342209/
- Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004; 45: 146–159. PubMed: https://pubmed.ncbi.nlm.nih.gov/14988485/
- Bonales-Alatorre E, Shabala S, Chen ZH, Pottosin I. Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa. Plant Physiol. 2013; 162: 940–952. PubMed: https://pubmed.ncbi.nlm.nih.gov/23624857/
- Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot. 2013; 112: 1209–1221. PubMed: https://pubmed.ncbi.nlm.nih.gov/24085482/
- Maathuis FJM. Sodium in plants: perception, signaling, and regulation of sodium fluxes. J Exp Bot. 2014; 65: 849–858. PubMed: https://pubmed.ncbi.nlm.nih.gov/24151301/
- Colmenero-Flores JM, Martínez G, Gamba G, Vázquez N, Iglesias DJ, et al. Identification and functional characterization of cation-chloride co-transporters in plants. Plant J. 2007; 50: 278–292. PubMed: https://pubmed.ncbi.nlm.nih.gov/17355435/
- Wegner LH, De Boer AH. Two inward K+ channels in the xylem parenchyma cells of barley roots are regulated by G-protein modulators through a membrane-delimited pathway. Planta. 1997; 203: 506–516.
- Yadav N, Shukla P, Jha A, Agarwal PK, Jha B. The SbSOS1 gene from the extreme halophyte Salicornia brachiate enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012; 12: 188. PubMed: https://pubmed.ncbi.nlm.nih.gov/23057782/
- Zhu M, Shabala L, Cuin TA, Huang X, Zhou M, et al. Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J Exp Bot. 2016; 67: 835–844. PubMed: https://pubmed.ncbi.nlm.nih.gov/26585227/
- Sunarpi, Horie T, Motoda J, Kubo M, Yang H, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005; 44: 928–938. PubMed: https://pubmed.ncbi.nlm.nih.gov/16359386/
- Huang S, Spielmeyer W, Lagudah ES, James RA, Platten JD, et al. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006; 142: 1718–1727. PubMed: https://pubmed.ncbi.nlm.nih.gov/17071645/
- Jaime-Pérez N, Pineda B, García-Sogo B, Atares A, Athman A, et al. The sodium transporter encoded by the HKT1;2 gene modulates sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017; 40: 658–671. PubMed: https://pubmed.ncbi.nlm.nih.gov/27987209/
- Kong X, Luo Z, Dong H, Eneji AE, Li W. Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J Exp Bot. 2012; 63: 2105–2116. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3295398/
- Rus A, Lee BH, Muñoz-Mayor A, et al. AtHKT1 Facilitates Na+ Homeostasis and K+ Nutrition in Planta. Plant Physiol. 2004; 136: 2500-2511. PubMed: https://pubmed.ncbi.nlm.nih.gov/15347798/
- Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, et al. The Na+ transporter AtHKT1;1 control retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 207; 30: 497–507. PubMed: https://pubmed.ncbi.nlm.nih.gov/17324235/
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003; 91: 503–527. PubMed: https://pubmed.ncbi.nlm.nih.gov/12646496/
- Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance, EMBO J. 2003; 22: 2004–2014. PubMed: https://pubmed.ncbi.nlm.nih.gov/12727868/
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet. 2005; 37: 1141–1146. PubMed: https://pubmed.ncbi.nlm.nih.gov/16155566/
- Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, et al. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2017; 91: 657–670. PubMed: https://pubmed.ncbi.nlm.nih.gov/28488420/
- Wang Y, Wu WH. Potassium transport and signaling in higher plants, Annu. Rev. Plant Biol. 2013; 64: 451–476. PubMed: https://pubmed.ncbi.nlm.nih.gov/23330792/
- Shi H, Ishitani M, Kim C, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci. U.S.A. 2000; 97: 6896–6901. PubMed: https://pubmed.ncbi.nlm.nih.gov/10823923/
- Shabala S, Shabala L, Van Volkenburgh E, Newman I. Effect of divalent cations on ion fluxes and leaf photochemistry in salinized barley leaves. J Exp Bot. 2005; 56: 1369–1378. PubMed: https://pubmed.ncbi.nlm.nih.gov/15809285/
- Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci. U.S.A. 2000; 97: 3730–3734. PubMed: https://pubmed.ncbi.nlm.nih.gov/10725382/
- Luan S, Lan W, Lee SC. Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL-CIPK network. Curr Opin. Plant Biol. 2009; 12: 339–346. PubMed: https://pubmed.ncbi.nlm.nih.gov/19501014/
- Halfter U, Ishitani M, Zhu JK. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl Acad Sci. U.S.A. 2000; 97: 3735–3740. PubMed: https://pubmed.ncbi.nlm.nih.gov/10725350/
- Shono M, Wada M, Hara Y, Fujii T. Molecular cloning of Na+-ATPase cDNA from a marine alga, Heterosigma akashiwo. Biochim. Biophys. Acta Biomembr. 2001; 1511: 193–199. PubMed: https://pubmed.ncbi.nlm.nih.gov/11248217/
- Lunde C, Drew DP, Jacobs AK, Tester M. Exclusion of Na+ via sodium ATPase (PpENA1) ensures normal growth of Physcomitrella patens under moderate salt stress. Plant Physiol. 2007; 144: 1786–1796. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1949878/
- Demidchik V, Maathuis FJM. Physiological roles of nonselective cation channels in plants: from salt stress to signaling and development. New Phytol. 2007; 175: 387–404.
- Zhang JL, Flowers TJ, Wang SM. Mechanisms of sodium uptake by roots of higher plants. Plant Soil. 2010; 326: 45–60.
- Demidchik V, Tester M. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002; 128: 379–387. PubMed: https://pubmed.ncbi.nlm.nih.gov/11842142/
- Maathuis FJ, Sanders D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 2001; 127: 1617–1625. PubMed: https://pubmed.ncbi.nlm.nih.gov/11743106/
- Oh DH, Zahir A, Yun DJ, Bressan RA, Bohnert HJ. SOS1 and halophytism. Plant Signal Behav. 2009; 4: 1081–1083. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2819520/
- Quan R, Wang J, Yang D, Zhang H, Zhang Z, et al. EIN3 and SOS2 synergistically modulate plant salt tolerance. Sci. Rep. 2017; 7: 44637. PubMed: https://pubmed.ncbi.nlm.nih.gov/28300216/
- Gong D, Guo Y, Schumaker KS, Zhu JK. The SOS3 family of calcium sensors and SOS2 family of protein kinases in Arabidopsis. Plant Physiol. 2004; 134: 919–926. PubMed: https://pubmed.ncbi.nlm.nih.gov/15020756/
- Bose J, Rodrigo-Moreno A, Lai D, Xie Y, Shen W, et al. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann Bot. 2015; 115: 481-494. PubMed: https://pubmed.ncbi.nlm.nih.gov/25471095/
- Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi H. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 2008; 53: 554–565. PubMed: https://pubmed.ncbi.nlm.nih.gov/17996020/
- Katiyar-Agarwal S, Zhu JJ, Kim K, Agarwal M, Fu X, et al. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proc Natl Acad Sci. U.S.A. 2006; 103: 18816–18821. PubMed: https://pubmed.ncbi.nlm.nih.gov/17023541/
- Sagor GHM, Zhang S, Kojima S, Simm S, Berberich T, et al. Reducing cytoplasmic polyamine oxidase activity in Arabidopsis increases salt and drought tolerance by reducing reactive oxygen species production and increasing defense gene expression. Front Plant Sci. 2016; 7: 214. PubMed: https://pubmed.ncbi.nlm.nih.gov/26973665/
- Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, et al. Overexpression of SOS (salt overly sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol Plant. 2009; 2: 22–31. PubMed: https://pubmed.ncbi.nlm.nih.gov/19529826/
- Yue Y, Zhang M, Zhang J, Duan L, Li Z. SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio. J Plant Physiol. 2012; 169: 255–261. PubMed: https://pubmed.ncbi.nlm.nih.gov/22115741/
- Leidi EO, Barragán V, Rubio L, El-Hamdaoui A, Ruiz MT, et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 2010; 61: 495–506.
- Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, et al. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell. 2011; 23: 3482–3497. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3203450/
- Barragan V, Leidi EO, Andres Z, Rubio L, De Luca A, et al. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell. 2012; 24: 1127–1142. PubMed: https://pubmed.ncbi.nlm.nih.gov/22438021/
- Liu X, Cai S, Wang G, Wang F, Dong F, et al. Halophytic NHXs confer salt tolerance by altering cytosolic and vacuolar K+ and Na+ in Arabidopsis root cell. Plant Growth Regul. 2017; 82: 333–351.
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, et al. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci. U.S.A. 1999; 96: 1480–1485. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC15488/
- Adem G, Roy SJ, Zhou M, Bowman JP, Shabala S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014; 14: 113. PubMed: https://pubmed.ncbi.nlm.nih.gov/24774965/
- Sandhu D, Cornacchione MV, Ferreira JFS, Suarez DL. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci Rep. 2017; 7: 42958. PubMed: https://pubmed.ncbi.nlm.nih.gov/28225027/
- Mullan DJ, Colmer TD, Francki MG. Arabidopsis-rice-wheat gene orthologues for Na+ transport and transcript analysis in wheat-L. elongatum aneuploids under salt stress. Mol Gen Genomics. 2007; 277: 199–212. PubMed: https://pubmed.ncbi.nlm.nih.gov/17103227/
- Silva P, Gerós H. Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signal. Behav. 2009; 4: 718–726. PubMed: https://pubmed.ncbi.nlm.nih.gov/19820346/
- Baisakh N, Ramanarao MV, Rajasekaran K, Subudhi P, Janda J, et al. Enhanced salt stress tolerance of rice plants expressing a vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from the halophyte grass Spartina alterniflora Löisel. Plant Biotechnol J. 2012; 10: 453–464. PubMed: https://pubmed.ncbi.nlm.nih.gov/22284568/
- Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, et al. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci. U.S.A. 2001; 98: 11444–11449. PubMed: https://pubmed.ncbi.nlm.nih.gov/11572991/
- Qui QS, Guo Y, Quintero FJ, Pardo JM, Schumaker KS, et al. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the Salt-Overly-Sensitive (SOS) pathway. J Biol Chem. 2004; 279: 207–215. PubMed: https://pubmed.ncbi.nlm.nih.gov/14570921/
- Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E. Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci. U.S.A. 2005; 102: 16107–16112. PubMed: https://pubmed.ncbi.nlm.nih.gov/16249341/
- Quan R, Lin H, Mendoza I, Zhang Y, Cao W, et al. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell. 2007; 19: 1415–1431. PubMed: https://pubmed.ncbi.nlm.nih.gov/17449811/
- Tang RJ, Yang Y, Yang L, Liu H, Wang CT, et al. Poplar calcineurin B-like proteins PtCBL10A and PtCBL10B regulate shoot salt tolerance through interaction with PtSOS2 in the vacuolar membrane. Plant Cell Environ. 2014; 37: 573–588. PubMed: https://pubmed.ncbi.nlm.nih.gov/23941462/
- Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, et al. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007; 52: 473–484. PubMed: https://pubmed.ncbi.nlm.nih.gov/17825054/
- Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol. 2017; 8: 509. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513949/
- White PG, Broadley MR. Chloride in soils and its uptake and movement with the plant. Ann Bot. 2001; 88: 967-988.
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008; 179: 945-963.
- Hechenberger M, Schwappah B, Fischer WN, Frommer WB, Jentsch TJ, et al. A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene distruption. J Biol Chem. 1996; 271: 33632-33638. PubMed: https://pubmed.ncbi.nlm.nih.gov/8969232/
- Diédhiou CJ, Golldack D. Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci. 2006; 170: 793-800.
- Xu G, Magen H, Tarchitzky J, Kafkafi U. Advances in chloride nutrition of plants. Adv. Agronom. 2000; 68: 97-150.
- De Angeli A, Thomine S, Franchisse JM, Ephritikhinea G, Gambale F, et al. Anions channels and transporters in plant cell membranes. FEBS Lett. 2007; 581: 2367-2374. PubMed: https://pubmed.ncbi.nlm.nih.gov/17434490/
- Diédhiou CJ. Mechanisms of salt tolerance: sodium, chloride and potassium homeostasis in two rice lines with different tolerance to salinity stress. PhD thesis 2006; University of Bielefeld, Germany.
- Li WYF, Wong FL, Tsai SN, Phang TH, Shao G, et al. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ. 2006; 29: 1122-1137. PubMed: https://pubmed.ncbi.nlm.nih.gov/17080938/
- Nakamura A, Fukuda A, Sakai S, Tanaka Y. Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol. 2006; 47: 32-42. PubMed: https://pubmed.ncbi.nlm.nih.gov/16249326/
- Hedrich R. Ion channels in plants. Physiol Rev. 2012; 92: 1777–1811. PubMed: https://pubmed.ncbi.nlm.nih.gov/23073631/
- Nieves-Cordones M, Aleman F, Martinez V, Rubio F. K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J Plant Physiol. 2014; 171: 688–695. PubMed: https://pubmed.ncbi.nlm.nih.gov/24810767/
- Li W, Xu G, Alli A, Yu. Plant HAK/KUP/KT K+ transporters: function and regulation. Semin Cell Dev. Biol. 2018; 74: 133–141. PubMed: https://pubmed.ncbi.nlm.nih.gov/28711523/
- Hamamoto S, Horie T, Hauser F, Deinlein U, Schroeder JI, et al. HKT transporters mediate salt stress resistance in plants: from structure and function to the field. Curr Opin Biotechnol. 2015; 32: 113–120. PubMed: https://pubmed.ncbi.nlm.nih.gov/25528276/
- Sze H, Chanroj S. Plant endomembrane dynamics: studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol. 2018; 177: 875–895. PubMed: https://pubmed.ncbi.nlm.nih.gov/29691301/
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Sci. 1998; 280: 918–921. PubMed: https://pubmed.ncbi.nlm.nih.gov/9572739/
- Dreyer I, Uozumi N. Potassium channels in plant cells. FEBS J. 2011; 278: 4293–4303. PubMed: https://pubmed.ncbi.nlm.nih.gov/21955642/
- Jeanguenin L, Alcon C, Duby G, Boeglin M, Cherel I, et al. AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 2011; 67: 570–582. PubMed: https://pubmed.ncbi.nlm.nih.gov/21518051/
- Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci. U.S.A. 2007; 104: 10726–10731. PubMed: https://pubmed.ncbi.nlm.nih.gov/17563365/
- Latz A, Mehlmer N, Zapf S, Mueller TD, Wurzinger B, et al. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs). Mol Plant. 2013; 6: 1274–1289. PubMed: https://pubmed.ncbi.nlm.nih.gov/23253603/
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005; 434: 404–408. PubMed: https://pubmed.ncbi.nlm.nih.gov/15772667/
- Hedrich R, Mueller TD, Becker D, Marten I. Structure and function of TPC1 vacuole SV channel gains shape. Mol. Plant 2018; 11: 764–775. PubMed: https://pubmed.ncbi.nlm.nih.gov/29614320/
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci. U.S.A. 2014; 111: 6497–6502. PubMed: https://pubmed.ncbi.nlm.nih.gov/24706854/
- Evans MJ, Choi WG, Gilroy S, Morris RJ. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016; 171: 1771–1784. PubMed: https://pubmed.ncbi.nlm.nih.gov/27261066/
- Greiner T, Ramos J, Alvarez MC, Gurnon JR, Kang M, et al. Functional HAK/KUP/KT-like potassium transporter encoded by chlorella viruses. Plant J. 2011; 68: 977–986. PubMed: https://pubmed.ncbi.nlm.nih.gov/21848655/
- Santa-Maria GE, Oliferuk S, Moriconi, JI. KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: a twenty years tale. J Plant Physiol. 2018; 226: 77–90. PubMed: https://pubmed.ncbi.nlm.nih.gov/29704646/
- Very AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, et al. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol. 2014; 171: 748–769. PubMed: https://pubmed.ncbi.nlm.nih.gov/24666983/
- Benito B, Haro R, Amtmann A, Cuin TA, Dreyer I. The twins K+ and Na+ in plants. J Plant Physiol. 2014; 171: 723–731. PubMed: https://pubmed.ncbi.nlm.nih.gov/24810769/
- Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, et al. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 2006; 11: 372–374. PubMed: https://pubmed.ncbi.nlm.nih.gov/16809061/
- Ma YC, Auge RM, Dong C, Cheng ZM. Increased salt tolerance with overexpression of cation/proton antiporter 1 genes: a meta-analysis. Plant Biotechnol J. 2017; 15: 162–173. PubMed: https://pubmed.ncbi.nlm.nih.gov/27383431/
- De Luca A, Pardo JM, Leidi EO. Pleiotropic effects of enhancing vacuolar K/H exchange in tomato. Physiol Plant. 2018; 163: 88–102. PubMed: https://pubmed.ncbi.nlm.nih.gov/29076168/
- Jiang XY, Leidi EO, Pardo JM. How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav. 2010; 5: 792–795. PubMed: https://pubmed.ncbi.nlm.nih.gov/20495345/
- Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci. U.S.A. 2014; 111: E1806–E1814. PubMed: https://pubmed.ncbi.nlm.nih.gov/24733919/
- Ahmad I, Maathuis FJ. Cellular and tissue distribution of potassium: physiological relevance, mechanisms and regulation. J Plant Physiol. 2014; 171: 708–714. PubMed: https://pubmed.ncbi.nlm.nih.gov/24810768/
- Yang T, Zhang S, Hu Y, Wu F, Hu Q, et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014; 166: 945–959. PubMed: https://pubmed.ncbi.nlm.nih.gov/25157029/
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, et al, Identification and disruption of a plant Shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998; 94: 647–655. PubMed: https://pubmed.ncbi.nlm.nih.gov/9741629/
- Johansson I, Wulfetange K, Porée F, Michard E, Gajdanowicz P, et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 2006; 46: 269–281. PubMed: https://pubmed.ncbi.nlm.nih.gov/16623889/
- Thompson MV, Zwieniecki MA. “The role of potassium in long distance transport in plants” in Vascular transport in plants. Eds. NM. Holbrook and MA. Zwieniecki (Burlington: Academic Press). 2005; 221–240.
- De Schepper V, De Swaef T, Bauweraerts I, Steppe K. Phloem transport: a review of mechanisms and controls. J Exp Bot. 2013; 64: 4839–4850. PubMed: https://pubmed.ncbi.nlm.nih.gov/24106290/
- Deeken R, Sanders C, Ache P, Hedrich R. Developmental and light-dependent regulation of a phloem-localised K+ channel of Arabidopsis thaliana. Plant J. 2000; 23: 285–290. PubMed: https://pubmed.ncbi.nlm.nih.gov/10929122/
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, et al. A Shaker-like K(+) channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell. 2000; 12: 837–851. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC149088/
- Gajdanowicz P, Michard E, Sandmann M, Rocha M, Correa LG, et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc Natl Acad Sci. U.S.A. 2011; 108: 864–869.
- Dreyer I, Michard E, Lacombe B, Thibaud JB. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or ‘leak’ current. FEBS Lett. 2001; 505: 233–239.
- Chérel I, Lefoulon C, Boeglin M, Sentenac H. Molecular mechanisms involved in plant adaptation to low K+ availability. J Exp Bot. 2014; 65: 833-848. PubMed: https://pubmed.ncbi.nlm.nih.gov/24293613/
- Cherel I, Michard E, Platet N, Mouline K, Alcon C, et al. Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell. 2002; 14: 1133–1146. PubMed: https://pubmed.ncbi.nlm.nih.gov/12034902/
- Michard E, Lacombe B, Porée F, Mueller-Roeber B, Sentenac H, et al. A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J Gen Physiol. 2005a; 126: 605–617. PubMed: https://pubmed.ncbi.nlm.nih.gov/16316977/
- Michard E, Dreyer I, Lacombe B, Sentenac H, Thibaud JB. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 2005b; 44: 783–797. PubMed: https://pubmed.ncbi.nlm.nih.gov/16297070/
- Han M, Wu W, Wu WH, Wang Y. Potassium transporter KUP7 Is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol Plant. 2016; 9: 437–446. PubMed: https://pubmed.ncbi.nlm.nih.gov/26851373/
- Zhang M, Cao Y, Wang Z, Wang ZQ, Shi J, et al. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 2018; 217: 1161–1176. PubMed: https://pubmed.ncbi.nlm.nih.gov/29139111/
- Kellermeier F, Armengaud P, Seditas TJ, Danku J, Salt DE, et al. Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell. 2014; 26: 1480–1496. PubMed: https://pubmed.ncbi.nlm.nih.gov/24692421/
- Engels C, Marschner H. Influence of the form of nitrogen supply on root uptake and translocation of cations in the xylem exudate of maize (Zea mays L). J Exp Bot. 1993; 44: 1695–1701.
- Rodenas R, Garcia-Legaz MF, Lopez-Gomez E, Martinez V, Rubio F, et al. NO3−, PO43− and SO42− deprivation reduced LKT1-mediated low-affinity K+ uptake and SKOR-mediated K(+) translocation in tomato and Arabidopsis plants. Physiol Plant. 2017; 160: 410–424. PubMed: https://pubmed.ncbi.nlm.nih.gov/28244226/
- Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell. 2008; 20: 2514–2528. PubMed: https://pubmed.ncbi.nlm.nih.gov/18780802/
- Drechsler N, Zheng Y, Bohner A, Nobmann B, von Wiren N, et al. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol. 2015; 169: 2832–2847. PubMed: https://pubmed.ncbi.nlm.nih.gov/26508776/
- Meng S, Peng JS, He YN, Zhang GB, Yi HY, et al. Arabidopsis NRT1.5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol Plant. 2016; 9: 461–470. PubMed: https://pubmed.ncbi.nlm.nih.gov/26732494/
- Li H, Yu M, Du XQ, Wang ZF, Wu WH, et al. NRT1.5/NPF7.3 functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. Plant Cell. 2017; 29: 2016–2026. PubMed: https://pubmed.ncbi.nlm.nih.gov/28739644/
- Rubio F, Fon M, Rodenas R, Nieves-Cordones M, Aleman F, et al. A low K+ signal is required for functional high-affinity K+ uptake through HAK5 transporters. Physiol Plant. 2014; 152: 558–570. PubMed: https://pubmed.ncbi.nlm.nih.gov/24716623/
- Nieves-Cordones M, Rodenas R, Lara A, Martinez V, Rubio F. The combination of K(+) deficiency with other environmental stresses: what is the outcome? Physiol Plant. 2019; 165: 264–276. PubMed: https://pubmed.ncbi.nlm.nih.gov/30187486/
- Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009; 138: 1184–1194. PubMed: https://pubmed.ncbi.nlm.nih.gov/19766570/
- Ragel P, Rodenas R, Garcia-Martin E, Andres Z, Villalta I, et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 2015; 169: 2863–2873. PubMed: https://pubmed.ncbi.nlm.nih.gov/26474642/
- Straub T, Ludewig U, Neuhauser B. The Kinase CIPK23 inhibits ammonium transport in Arabidopsis thaliana. Plant Cell. 2017; 29: 409–422. PubMed: https://pubmed.ncbi.nlm.nih.gov/28188265/
- Dubeaux G, Neveu J, Zelazny E, Vert G. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol Cell. 2018; 69: 953–964.e955. PubMed: https://pubmed.ncbi.nlm.nih.gov/29547723/
- Sun J, Bankston JR, Payandeh J, Hinds TR, Zagotta WN, et al. Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature. 2014; 507, 73–77. PubMed: https://pubmed.ncbi.nlm.nih.gov/24572362/
- D’yakova EV, Rakitin AL, Kamionskaya AM, Baikov AA, Lahti R, et al. A study of the effect of expression of the gene encoding the membrane H+-pyrophosphatase of Rhodospirillum rubrum on salt resistance of transgenic tobacco plants. Doklady Biol Sci. 2006; 409: 346–348.
- Gao F, Gao Q, Duan X, Yue G, Yang A, et al. Cloning of an H+-PPase gene from Thellungiella halophila and its heterologous expression to improve tobacco salt tolerance. J Exp Bot. 2006; 57: 3259–3270. PubMed: https://pubmed.ncbi.nlm.nih.gov/16940040/
- Li X, Guo C, Gu J, Duan W, Zhao M, et al. Overexpression of VP, a vacuolar H+-pyrophosphatase gene in wheat (Triticum aestivum L.), improves tobacco plant growth under Pi and N deprivation, high salinity, and drought. J Exp Bot. 2014; 65: 683–696. PubMed: https://pubmed.ncbi.nlm.nih.gov/24474810/
- He C, Yan J, Shen G, Fu L, Holaday AS, et al. Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the field. Plant Cell Physiol. 2005; 46: 1848–1854. PubMed: https://pubmed.ncbi.nlm.nih.gov/16179357/
- Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol Breed. 2006; 17: 341–353.
- Bhaskaran S, Savithramma DL. Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot. 2011; 62: 5561–5570. PubMed: https://pubmed.ncbi.nlm.nih.gov/21841179/
- Peleg Z, Apse MP, Blumwald E. Engineering salinity and water stress tolerance in crop plants: getting closer to the field. Adv Bot Res. 2011; 57: 405–443.
- Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M, et al. Plant NHX cation/proton antiporters. Plant Signal. Behav. 2009; 4: 265–276. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2664485/
- Oh DH, Gong Q, Ulanov A, Zhang Q, et al. Sodium stress in the halophyte Thellungiella halophila, and transcriptional changes in a thsos1-RNA iterference line. J Integr Plant Biol. 2007; 49: 1484–1496.
- Pardo JM, Cubero B, Leidi EO, Quintero FJ. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006; 57: 1181–1199. PubMed: https://pubmed.ncbi.nlm.nih.gov/16513813/
- Olías R, Eljakaoui Z, Li J, De Morales PA, Marín-Manzano MC, et al. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009; 32: 904–916. PubMed: https://pubmed.ncbi.nlm.nih.gov/19302170/
- Wu SJ, Ding L, Zhu JK. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 1996; 8: 617–627. PubMed: https://pubmed.ncbi.nlm.nih.gov/12239394/
- Shi H, Lee BH, Wu SJ, Zhu JK. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol. 2003; 21: 81–85. PubMed: https://pubmed.ncbi.nlm.nih.gov/12469134/
- Feki K, Quintero FJ, Khoudi H, Leidi EO, Masmoudi K, et al. A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Rep. 2014; 33: 277–288. PubMed: https://pubmed.ncbi.nlm.nih.gov/24150094/
- De Boer AH, Volkov V. Logistics of water and salt transport through the plant: structure and functioning of the xylem. Plant Cell Environ. 2003; 26: 87-101.
- Guo KM, Babourina O, Rengel Z. Na+/H+ antiporter activity of the SOS1 gene: life time imaging analysis and electrophysiological studies on Arabidopsis seedlings. Physiol Plant. 2009; 137: 155–165. PubMed: https://pubmed.ncbi.nlm.nih.gov/19758408/
- Mäser P, Eckelman B, Vaidyanathan R, Horie T, Fairbairn DJ, et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002; 531: 157–161. PubMed: https://pubmed.ncbi.nlm.nih.gov/12417304/
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, et al. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type- specific alteration of Na+ transport in Arabidopsis. Plant Cell. 2009; 21: 2163–2178. PubMed: https://pubmed.ncbi.nlm.nih.gov/19584143/
- Plett D, Safwat G, Gilliham M, Møller IS, Roy S, et al. Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS ONE 2010; 5: e12571. PubMed: https://pubmed.ncbi.nlm.nih.gov/20838445/
- Mian A, Oomen RJ, Isayenkov S, Sentenac H, Maathuis FJ, et al. Over-expression of an Na+- and K+- permeable HKT transporter in barley improves salt tolerance. Plant J. 2011; 68: 468–479. PubMed: https://pubmed.ncbi.nlm.nih.gov/21749504/
- Horie T, Hauser F, Schroeder JI. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009; 14: 660–668. PubMed: https://pubmed.ncbi.nlm.nih.gov/19783197/
- Almeida P, Katschnig D, deBoer AH. HKT transporters-state of the art. Int J Mol Sci. 2013; 14: 20359–20385. PubMed: https://pubmed.ncbi.nlm.nih.gov/24129173/
- Maathuis FJM, Ahmad I, Patishtan J. Regulation of Na+ fluxes in plants. Front Plant Sci. 2014; 5: 467. PubMed: https://pubmed.ncbi.nlm.nih.gov/25278946/
- McAllister CH, Beatty PH, Good AG. Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol J. 2012; 10: 1011–1025. PubMed: https://pubmed.ncbi.nlm.nih.gov/22607381/
- Wang YY, Hsu PK, Tsay YF. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012; 17: 458–467. PubMed: https://pubmed.ncbi.nlm.nih.gov/22658680/
- Kiba T, Feria-Bourrellier AB, Lafouge F, Lezhneva L, Boutet-Mercey S, et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012; 24: 245–258. PubMed: https://pubmed.ncbi.nlm.nih.gov/22227893/
- Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, et al. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci. USA. 2005; 102: 13693–13698. PubMed: https://pubmed.ncbi.nlm.nih.gov/16157886/
- Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010; 18: 927–937. PubMed: https://pubmed.ncbi.nlm.nih.gov/20627075/
- Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, et al. Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci. U.S.A. 2011; 108: 18524–18529. PubMed: https://pubmed.ncbi.nlm.nih.gov/22025711/
Figures:
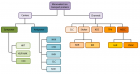
Figure 1
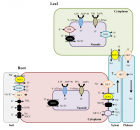
Figure 2
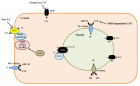
Figure 3
Similar Articles
-
Bacillus amyloliquefaciens as a plant growth promoting bacteria with the interaction with of grass salt Distichlis palmeri (Vasey) under field conditions, in desert of Sonora, MexicoRueda Puente Edgar O*,Ruiz Alvarado Cristina,Jesús Borboa Flores,Victor Cardenas Salazar,Soto Ortiz Roberto,Lourdes Díaz Cervantes. Bacillus amyloliquefaciens as a plant growth promoting bacteria with the interaction with of grass salt Distichlis palmeri (Vasey) under field conditions, in desert of Sonora, Mexico. . 2018 doi: 10.29328/journal.jpsp.1001021; 2: 059-067
-
Ion transporters and their molecular regulation mechanism in plantsFaiçal Brini*,Walid Saibi. Ion transporters and their molecular regulation mechanism in plants. . 2021 doi: 10.29328/journal.jpsp.1001058; 5: 028-043
-
Impact of Biofertilizers & Different doses of NPK on Growth and Photosynthetic Pigments of Okra Plant (Abelmoschus Esculentus L. Moench)Reena Kujur*, Heba I Khan, Eugenia P Lal, Lalit Kumar Verma. Impact of Biofertilizers & Different doses of NPK on Growth and Photosynthetic Pigments of Okra Plant (Abelmoschus Esculentus L. Moench). . 2023 doi: 10.29328/journal.jpsp.1001112; 7: 092-096
-
The Use of Thioxopyrimidine Derivatives as New Regulators of Growth and Photosynthesis of BarleyTsygankova VA*, Andrusevich YaV, Vasylenko NM, Kopich VM, Solomyannyi RM, Popilnichenko SV, Kozachenko OP, Pilyo SG, Brovarets VS. The Use of Thioxopyrimidine Derivatives as New Regulators of Growth and Photosynthesis of Barley. . 2024 doi: 10.29328/journal.jpsp.1001139; 8: 090-099
-
Plant growth, Yield and Leaf Nutritional value of Jute (Corchorus olitorius L.) as Influenced by Banana Peel levels under Salt Stress conditions in Coastal region of CameroonMathias Julien Hand*,Chimène Fanta Abib,Kingsley Mbi Tabi,Alphonse Ervé Nouck,Libert Brice Tonfack,Victor Désiré Taffouo,Emmanuel Youmbi. Plant growth, Yield and Leaf Nutritional value of Jute (Corchorus olitorius L.) as Influenced by Banana Peel levels under Salt Stress conditions in Coastal region of Cameroon. . 2024 doi: 10.29328/journal.jpsp.1001145; 8: 131-140
Recently Viewed
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001099; 8: 075-077
-
High-Performance Liquid Chromatography (HPLC): A reviewAbdu Hussen Ali*. High-Performance Liquid Chromatography (HPLC): A review. Ann Adv Chem. 2022: doi: 10.29328/journal.aac.1001026; 6: 010-020
-
Drug Rehabilitation Centre-based Survey on Drug Dependence in District Shimla Himachal PradeshKanishka Saini,Palak Sharma,Bhawna Sharma*,Atul Kumar Dubey,Muskan Bhatnoo,Prajkta Thakur,Vanshika Chandel,Ritika Sinha. Drug Rehabilitation Centre-based Survey on Drug Dependence in District Shimla Himachal Pradesh. J Addict Ther Res. 2025: doi: 10.29328/journal.jatr.1001032; 9: 001-006
-
Poly-dopamine-Beta-Cyclodextrin Modified Glassy Carbon Electrode as a Sensor for the Voltammetric Detection of L-Tryptophan at Physiological pHMohammad Hasanzadeh*,Nasrin Shadjou,Sattar Sadeghi,Ahad Mokhtarzadeh,Ayub karimzadeh. Poly-dopamine-Beta-Cyclodextrin Modified Glassy Carbon Electrode as a Sensor for the Voltammetric Detection of L-Tryptophan at Physiological pH . J Forensic Sci Res. 2017: doi: 10.29328/journal.jfsr.1001001; 1: 001-009
-
WMW: A Secure, Web based Middleware for C4I Interoperable ApplicationsNida Zeeshan*. WMW: A Secure, Web based Middleware for C4I Interoperable Applications. J Forensic Sci Res. 2017: doi: 10.29328/journal.jfsr.1001002; 1: 010-017
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."