Abstract
Research Article
Micropropagation and cytological studies of Aole vera Linn
Sweety Majumder*, Animesh Biswas and Mohammad Mahbubur Rahman
Published: 30 September, 2022 | Volume 6 - Issue 3 | Pages: 126-132
Aloe vera Linn. is an essential medicinal plant. In this present research work, a protocol of
in vitro regeneration and karyomorphological analysis of Aloe vera was developed using different concentrations and compositions of media. Shoot apices of field-grown plants were used as explant and aseptically cultured on Murashige and Skoog (MS) medium fortified with different concentrations and combinations of auxins (IAA and NAA) and cytokinins (BAP and Kn). The highest number of multiple shoot buds (4.36 ± 0.07) was obtained from MS + 2.0 mg/l BAP + 1.0 mg/l IAA and induced shoot buds underwent rapid elongation (4.24 ± 0.06 cm) on the same medium composition. Half strength MS media with 2.0 mg/l IBA was suitable for induction and proliferation (6.31 ± 0.05) of roots and 95% of plantlets were acclimatized to field conditions successfully. Somatic chromosome numbers of mother and in vitro grown plants were confirmed to be 2n = 14. Chromosome length ranged from 4.28 - 13.74 µm in the naturally grown plants and 4.46 - 14.1 µm for in vitro grown plants. The total form percent (TF%) of mother and in vitro grown plants was 41.69% and 42.23%, respectively. The karyotype formula of in vivo grown plants was 2n = 14 = 4Lsm + 6Mm + 4Sm, whereas that of the micropropagated plants was 2n = 14 = 4Lsm + 4Mm + 6Sm. The frequency of the chromosome having arm more than 2:1 was 0.08 for mother plants and 0.15 for in vitro grown plants. Therefore, the karyotype of both plants falls into the 2B symmetrical type based on Stebbins classification (1971).
Read Full Article HTML DOI: 10.29328/journal.jpsp.1001085 Cite this Article Read Full Article PDF
Keywords:
Aloe vera; Explant; In vitro propagation; Chromosome; Ideogram; Karyotype
References
- Grindlay D, Reynolds T. The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol. 1986 Jun;16(2-3):117-51. doi: 10.1016/0378-8741(86)90085-1. PMID: 3528673.
- Singh RP, Dhanalakshmi S, Rao AR. Chemomodulatory action of Aloe vera on the profiles of enzymes associated with carcinogen metabolism and antioxidant status regulation in mice. Phytomedicine. 2000 Jun;7(3):209-19. doi: 10.1016/S0944-7113(00)80006-9. PMID: 11185732.
- Davis RH. Aloe vera- A scientific approach. Vantage Press Inc, New York. 1997; 290–306.
- Bozzi A, Perrin C, Austin S. “Quality and authenticity of commercial aloe vera gel powders. Food Chemistry. 2007; 103(1): 22–30.
- Lawless J, Allan J. The Clinical Composition of Aloe vera, In: Aleo vera natural wonder cure. Thorsons, Publishing Ltd, London, United Kingdom. 2000; 161-171.
- Campestrini LH,Kuhnen L, Lemos PMM, Bach DB, Dias PF, Maraschin M. Cloning protocol of Aloe veraas study case for tailormade biotechnology to small farmers. Journal of Technology Management and Innovation. 2000; 1:76-7
- Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999 Dec 15;68(1-3):3-37. doi: 10.1016/s0378-8741(99)00085-9. PMID: 10624859.
- Yadav K, Singh N. Micropropagation ofSpilanthes acmella Murr. An important medicinal plant. National Sciences. 2010; 8: 4-
- Bhojwani SS, Razdan MK. Plant tissue culture: theory and practice. Elsevier. 1986.
- Baksha R, Jahan MAA, Rahima K. Micropropagation of Aloe barbadensis through In vitro culture of shoot tip explants. Plant Tissue Culture and Biotechnology. 2005; 152:121-126.
- Singh B, Sood N. Significance of explant preparation and sizing in Aloe vera-A highly efficientmethod for in vitro multiple shoot induction. Scientia Horticulturae. 2009; 122:146-151, 2009.
- Natali L, Sanchez I, Cavallini A. In vitro culture of Aloe barbadensis Mill: micropropagation from vegetative meristems. Plant Cell, Tissue and Organ Culture. 1990; 20:71-74.
- Samadder T, Nath S, Halder M, B. Sil, Roychowdhury D, Sen S, Sumita S. Karyotype analysis of three important traditional Indian medicinal plants Bacopa monnieri, Tylophora indica, and Withania somnifera. Nucleus; 55:17-20.
- Young HA, Sarath G, Tobias CM. Karyotype variation is indicative of subgenomic and ecotypic differentiation in switchgrass. BMC Plant Biol. 2012 Jul 26;12:117. doi: 10.1186/1471-2229-12-117. PMID: 22834676; PMCID: PMC3492167.
- Levan A, Fredga K, Sandberg AA. Nomenclature for Centromeric Position on Chromosomes. Hereditas. 1964; 52:201-220.
- Huziwara Y. Karyotypic analysis in some genera of compositae VIII. Further studies on the chromosomes of American Journal of Botany. 1982; 49:116-119
- Stebbins GL. Longevity, habitat. and release of genetic variability in the higher plants. Cold Spring Harb Symp Quant Biol. 1958;23:365-78. doi: 10.1101/sqb.1958.023.01.035. PMID: 13635568.
- Ahmed A, Omar H. In-vitropropagation of the multipurpose Egyptian medicinal plant Pimpinella anisum. Egyptian Pharmaceutical Journal. 2019; 18( 3):254-262.
- Manivannan A, Soundararajan P, Park YG, Jeong BR. In Vitro Propagation, Phytochemical Analysis, and Evaluation of Free Radical Scavenging Property of Scrophularia kakudensis Franch Tissue Extracts. Biomed Res Int. 2015;2015:480564. doi: 10.1155/2015/480564. Epub 2015 Nov 16. PMID: 26649304; PMCID: PMC4663745.
- Kirillov V, Pathak A, Stikhareva T, Ercisli S, Daulenova M, Kazangapova N, Rakhimzhanov A. “In vitro propagation and ex vitro rooting of Euonymus verrucosus (Celastraceae) - a rare species of Kazakhstan flora on the southern border of its areal. Journal of Forest Research. 2022; 27(4):289-296.
- Nazir U, Gul Z, Shah G, Khan N. Interaction Effect of Auxin and Cytokinin on in VitroShoot Regeneration and Rooting of Endangered Medicinal Plant Valeriana jatamansi Jones through Tissue Culture. American Journal of Plant Sciences. 2022; 13:23-240.
- Singh NM, Chanu LA, Devi YP, Singh WRC, Singh HB. Micropropagation-an in vitro technique for the conservation of Alpinia galangal. Advances in Applied Science Research. 2014; 5(3):259-263.
- Akın B, Çetin B, Bingöl NA. In vitro propagation of wetland medicinal plant Lythrum salicaria Celal Bayar University Journal of Science. 2018; 14(4): 369-372.
- Reshi KNA, Sudarshana MS, Girish HV. In vitro micropropagation of Rhinacanthus nasutus International Journal of Biodiversity and Conservation. 2018; 10(9):357-364.
- Lijalem T, Feyissa T. In vitro propagation of Securidaca longipedunculata (Fresen) from shoot tip: an endangered medicinal plant. J Genet Eng Biotechnol. 2020 Jan 20;18(1):3. doi: 10.1186/s43141-019-0017-0. PMID: 31956941; PMCID: PMC6970091.
- Zayova EG, Geneva MP, Georgieva KDM, Hristozkova MG, Stancheva IV. Impact of plant growth regulators on Greek oregano micropropagation and antioxidant activity. Biosciences Biotechnology Research Asia. 2019; 16(2):297-305.
- Aggarwal D, Upadhyay SK, Kumar K, Sehrawat N, Tuli HS, Singh R. Effects of plant growth regulators on in vitro propagation of economically important ornamental plant Rosa hybrida Asian Journal of Biological and Life Sciences. 2020; 9(2):227-233.
- Gopu C, Chakilam CS, Chirumamilla P, Vankudoth S, Taduri S. Rapid in vitro adventitious rooting and proliferation by leaf and nodal cultures of Momordica cymbalaria Journal of Applied Biology and Biotechnology. 2020; 8(02):103–107.
- Zayova E, Petrova M, Dimitrova L, Vasilevska-Ivanova R, Stoeva D. Effectof different auxins on in vitro rooting of Paulownia elongata propagated plants. Genetics and Plant Physiology. 2014; 4(3–4):155–162.
- Priyadarshni M, Arunima M, Kumara R, Shukla LN. Tissue culture studies of Heliotropium indicum an important medicinal herb for callus induction and micro propagation. International Journal of Research and Analytical Reviews. 2014; 1(4):618-625.
- Xin-Hua Z, Silva AT, Ma G. Karyotype analysis of Santalum album Caryologia. 2010; 63(2):142-148.
- Zhao C, Li F, Gao S. Research on Chromosome Karyotype Analysis of Plumbago auriculata. Open Access Library Journal. 2014; 1:1-7.
- Biswas and M. M. Rahman, “Karyotype analysis of Thespesia lampas (Cav.) Dalz. & Gibs. from Bangladesh,” Journal of Pharmacognosy and Phytochemistry. 2017; 6: 4;1316-1317.
- Xu J, Yin Z, Funamoto T, Peng H. First report of chromosome numbers and karyotypes of two monotypic genera endemic to eastern Asia: Brachystemma (Caryophyllaceae) and Craspedolobium (Fabaceae). Nordic Journal of Botany. 2011; 29:200-203.
- Yan QJ, Zou LJ, Tian TT, Wang L, Shen XL, Luo MH. [Karyotype analysis of three Lonicera species growth in Sichuan]. Zhong Yao Cai. 2014 Mar;37(3):384-7. Chinese. PMID: 25174099.
Figures:

Figure 1

Figure 2
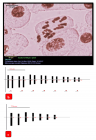
Figure 3
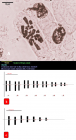
Figure 4
Similar Articles
-
Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformantsShu Wei*,Muhammad Abdullah,Ferdinand L Shamalla,Mohammad M Rana. Wild-type Agrobacterium rhizogenes-mediated gene transfer in plants: Agrobacterium virulence and selection of transformants. . 2017 doi: 10.29328/journal.jpsp.1001005; 1: 044-051
-
Genetic variability, divergence, and path coefficient analysis of yield and yield related traits of Durum wheat (Triticum turgidum l. var. Durum) genotypes at Jamma district, south wollo zone, amhara region, EthiopiaHaile Tefera*. Genetic variability, divergence, and path coefficient analysis of yield and yield related traits of Durum wheat (Triticum turgidum l. var. Durum) genotypes at Jamma district, south wollo zone, amhara region, Ethiopia. . 2022 doi: 10.29328/journal.jpsp.1001078; 6: 075-083
-
Micropropagation and cytological studies of Aole vera LinnSweety Majumder*,Animesh Biswas,Mohammad Mahbubur Rahman. Micropropagation and cytological studies of Aole vera Linn. . 2022 doi: 10.29328/journal.jpsp.1001085; 6: 126-132
-
Cold Atmospheric Pressure Plasma Jet and Plasma Lamp Interaction with Plants: Electrostimulation, Reactive Oxygen and Nitrogen Species, and Side EffectsAlexander G Volkov*, Jewel S Hairston, Darayas Patel, Sergey Sarkisov. Cold Atmospheric Pressure Plasma Jet and Plasma Lamp Interaction with Plants: Electrostimulation, Reactive Oxygen and Nitrogen Species, and Side Effects. . 2023 doi: 10.29328/journal.jpsp.1001110; 7: 081-088
Recently Viewed
-
COPD and low plasma vitamin D levels: Correlation or causality?Luca Gallelli*, Erika Cione,Stefania Zampogna, Gino Scalone. COPD and low plasma vitamin D levels: Correlation or causality? . J Pulmonol Respir Res. 2018: doi: 10.29328/journal.jprr.1001008; 2: 011-012
-
Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A ReviewDivya Khorwal*, GK Mathur, Umema Ahmed, SS Daga. Environmental Factors Affecting the Concentration of DNA in Blood and Saliva Stains: A Review. J Forensic Sci Res. 2024: doi: 10.29328/journal.jfsr.1001057; 8: 009-015
-
Markov Chains of Molecular Processes of Biochemical MaterialsOrchidea Maria Lecian*. Markov Chains of Molecular Processes of Biochemical Materials. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001076; 7: 001-005
-
Generation of Curved Spacetime in Quantum FieldSarfraj Khan*. Generation of Curved Spacetime in Quantum Field. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001077; 7: 006-009
-
Optimizing Milk Safety: Applying Nuclear Techniques in X-ray Fluorescence Spectroscopy for Heavy Metal Quantification in Powdered Milk Consumed in SenegalPapa Macoumba Faye*, Djicknack Dione, Oumar Ndiaye, Moussa Hamady SY, Nogaye Ndiaye, Alassane Traore, Ababacar Sadikhe Ndao. Optimizing Milk Safety: Applying Nuclear Techniques in X-ray Fluorescence Spectroscopy for Heavy Metal Quantification in Powdered Milk Consumed in Senegal. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001078; 7: 010-015
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."