Research Article
Impact of Calcium Phosphate Nanoparticles on Rice Plant

Hrishikesh Upadhyaya1,2*, Lutfa Begum1, Bishal Dey1, P K Nath1, and S K Panda3
1Department of Botany and Biotechnology, Karimganj College, Karimganj 788710, Assam, India
2Department of Botany, Cotton College State University, Guwahati-781001, Assam, India
3Plant Molecular Biotechnology Laboratory, Department of Life Science and Bioinformatics, Assam University , Silchar 788011, Assam, India
*Address for Correspondence: Hrishikesh Upadhyaya, Department of Botany, Cotton College State University, Guwahati-781001, Assam, India, E-mail: hkupbl_au@rediffmail.com
Dates: Submitted: 21 December 2016; Approved: 20 February 2017; Published: 21 February 2017
How to cite this article: Upadhyaya H, Begum L, Dey B, Nath PK, Panda SK. Impact of Calcium Phosphate Nanoparticles on Rice Plant. J Plant Sci Phytopathol. 2017; 1: 001-010.
DOI: 10.29328/journal.jpsp.1001001
Copyright: © 2017 Upadhyaya H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
Calcium phosphates are of great interest in medicine, biology, agriculture and materials sciences. The present study evaluates the effect of calcium phosphates nanoparticles on biochemical changes in rice. Nanoparticles increased the growth rate and affect the physiology of the plant. Calcium phosphate nanoparticles may help in the formulation of new nano growth promoter and nano-fertilizers for agricultural use. Therefore, it could potentially help in reduction of the quantity of fertilizer applied to crops and contributing to precision farming as it reduces fertilizer wastage and in turn environmental pollution due to agricultural malpractices. However, detail physiological and molecular understanding of its impact on rice crop plant is needed in future to validate its prospective application in agriculture.
INTRODUCTION
Nanotechnology is a very promising field of science and technology that has the potential to open up new applications in the field of agriculture and biotechnology [1]. Ultrafine particles are between 1 and 100nm in size. Nanoparticles can serve as magic bullets containing herbicides, chemicals or genes which target plant parts to release the content [2]. In agriculture, fertilizers are very important for plant growth and development, most of the applied fertilizer are rendered unavailable to the plants due to many factors such as leaching, degradation by photolysis, hydrolysis and degradation. Hence it is necessary to minimize nutrient losses in fertilization and to increase the crop yield through the exploitation of new application with the help of nanotechnology and nanomaterials. Nanoparticles have unique physicochemical properties and the potential to boost the plant metabolism [3].
Calcium is an essential plant nutrient. It plays many important role in plant like, participate in the metabolic process of another nutrient uptake, promotes proper plant cell elongation, strengthen cell wall, participate in an enzymatic and hormonal process. High levels of Ca2+ can alter both growth and Na+ exclusion of plant roots exposed to NaCl stress [4]. In addition, root supplied with high levels of Ca2+ are often maintaining their K+ concentration, whereas root supplied with low Ca2+ frequently cannot [5-7]. The adequate level of Ca2+ is required in the external medium to maintain the selectivity and integrity of cell membrane. Calcium competes with other positively charged ion, such as Na+, K+, Mg2+ and thus applying too much of these positively charged ions might decrease calcium uptake by plants.
Most biotic and abiotic stresses elicit an increase in cytosolic free calcium concentrations [8-11]. Specific responses to different stimuli could be achieved through variations in the amplitude, duration, location, and frequency of these Ca2+-spikes [12]. As Ca2+ is ubiquitous in stress signaling, it may be an important node at which crosstalk between pathways can occur. Four major families of calcium-binding proteins have been identified in plants: calmodulins, calmodulin-like proteins, calcineurin B-like proteins, and calcium-dependent protein kinases (CDPKs) [10,13,14]. CDPKs have a conserved molecular structure, consisting of a variable N terminal domain, fused to a Ser/Thr kinase domain, and a CDPK activation domain (CAD). The CAD is formed from a pseudosubstrate region (sometimes referred to as the ‘inhibitory junction domain’) and a Ca2+-binding domain, highly homologous to calmodulin. Thus Ca is an important component of cellular signaling system. Phosphorous is also an essential element required for the energy storage and transfer within plants. It is a major component in ATP, the molecule that provides ‘energy’to the plant for such processes as photosynthesis, protein synthesis, nutrition translocation, nutrient uptake respiration and transfer of genetic characteristics from one generation to next. Phosphorous has been observed to increase root growth and influence early maturity, straw strength, crop quality and diseases resistance. Severe foliar P deficiency symptoms are occasionally observed in commercial rice fields. Phosphorous is classified as a major nutrient required by crops in relatively large amounts. The total P concentration in agricultural crops generally varies from 0.1 to 0.5 percent.
Nanotechnology has wide application in different fields of science. The use of nanoparticles in the growth of plants and for the control of plant diseases is a recent practice [15-18]. However, whether beneficial or harmful to plant growth is an unresolved issue. Various studies had revealed a numbers of the good attempt to understand the impact of nanoparticles on the growth of plants [17,18]. Lieu and Lal [19] suggested positive impact of NP on plant with its potential to be used as future nanofertilizer. Rane et al., [20] reported that calcium phosphate nanoparticles (CaPO4 NPs) exhibit synergistic growth promotion, root proliferation and vitality improvement properties along with endosymbiotic and arbuscular mycorrhizal fungi, which after further field trials can be developed as a cost effective nano-fertilizer with pronounced efficiency. The present study provides new information on the effect of calcium phosphate nanoparticles on growth and biochemical changes of the rice plant. The proposed research is designed, therefore, to understand the physiological impact of calcium phosphate nanoparticles on rice under laboratory conditions.
MATERIALS AND METHODS
Plant material and growth conditions
Rice (Oriza sativaL) seeds (Var. Kopilee) were collected from Regional Agricultural Research Station (RARS), Akbarpur, Karimganj, Assam. The viable seeds were surface sterilized with 0.1% HgCl2 and washed thoroughly with distilled water. The concentration, 0.1% HgCl2 is used to decontaminate seed surface. The seeds were placed in a petriplate containing moister filter paper and kept for germination in seed germination chamber at 28°C. On the third day of germination uniformly germinated seeds were transplanted into plastic cups (180ml) containing Hogland solution of pH at 6.8. On the fifth day of transfer the seeds were subjected to different treatment. The seedlings were treated with calcium phosphate nano-particle, 10mg/L, 20mg/L, 50mg/L respectively. The calcium phosphate nanoparticles used were purchased from SRL India, and its size is 30 nm. Seedlings grown in normal Hoagland is considered as ‘control’.
Plant growth
After 48h of treatment, plants were sampled for various physiological and biochemical analysis such as length, fresh mass and dry mass of roots and shoots and using centimeter ruler root and shoot length were measured and were separated and fresh mass was taken and then oven dried at 80°C for 48h to estimate the dry mass and expressed in gram per plant. Total fresh mass and dry mass of plant were estimated by adding root and shoot, fresh mass and dry mass respectively.
ROS assay
Estimation of superoxide anion (O-2) in rice plant subjected to Ca3(PO4)2 NP (0mg/L, 10mg/L, 20mg/L, 50mg/L respectively) was done as per method of Doke [21]. The superoxide anion (O-2) content was assayed spectrophotometrically and thus measuring the reduction of nitro blue tetrazolium (NBT). Ten leaf disks (diameter=3mm) were immersed 2ml of the mixture containing 0.01M sodium phosphate buffer (7.8), 0.05% NBT, and 10Mm NaN3 in a beaker. After incubating the mixture for 60min at room temperature, 1.5ml of the reaction solution was transferred into micro-centrifuged tube and was heated at 85°C for 15minutes. Then the solution was cooled and its absorbance at 580nm was measured. For determining superoxide anion (O-2) generation, NBT reducing activity was expressed as the increase in A580 per hour per gram of dry weight. For determining H2O2, 100mg plant tissues were homogenized in 2ml of 0.05M phosphate buffer at pH 7.4 and the homogenized was centrifuged at 15000 g for 25min. Then 1.5ml of the supernatant was added with 0.5ml of 0.1% TiCl2 in 50% v/v H2SO4 and the mixture was again centrifuged at 15000 g for 15min and then the absorbance was measured at 410nm using Biospectrometer Basic, Eppendorf, India [22].
Lipid peroxidation assay
To estimate malondialdehyde (MDA) content 50mg of plant tissue were homogenized in 2ml of 0.25% TBA in 10% TCA. Samples were heated for 30min at 95°C and immediately cooled in a ice bath. The samples were then centrifuged for 10 min at 10000 rpm. The supernatant was collected and was taken at 532nm and 600nm [23] using Biospectrometer Basic, Eppendorf, India.
Antioxidants metabolite assay
Estimation of ascorbate and glutathione in the control and treated growing seedlings of rice was also carried out. Glutathione was extracted and estimated by following the methods of Griffith [24] by taking 100 mg plant tissues that were homogenized in 5% m-phosphoric acid and the homogenate was centrifuged at 18000g for 10 min. The supernatant (1ml) was then neutralized with 0.5 ml of 0.5M potassium phosphate buffer (pH 7.5) and total glutathione was measured by adding 1ml of neutralized supernatant to a standard solution mixture consisting of 0.5ml of 0.1 M potassium phosphate buffer (pH 7.5) containing EDTA, 0.2ml of 6mM DTNB, 0.1 ml of 2mM NADPH and 1 ml of 1unit GR (Sigma chemicals, St. Louis, MO, USA). The change in absorbance at 412 nm was read. For the extraction and estimation of ascorbate, the method of Oser [25] was used. The reaction mixture consisted of 2ml of 0.15N H2SO4, 2ml of sodium molybdate, 1ml of 1.5mM Na2HPO2 and 1ml of tissue extract. The reaction mixture was incubated at 60°C in water bath for 40 min and cooled and centrifuged at 3000g for 10 min, and then the absorbance was measured at 660 nm using Biospectrometer Basic, Eppendorf, India.
Antioxidant enzyme analysis
For extracting and estimating various enzyme activities, 100 mg of plant tissue were homogenized in potassium phosphate buffer (pH 6.8, 0.1M) containing 0.1mM Ethylenediaminetetraacetic acid (EDTA), 1% polyvinylpyrrolidone (PVP) and 0.1mM phenylmethanesulfonylfloride (PMSF) in a pre-chilled mortar and pestle. The extract was centrifuged at 4°C for 15mins at 13000 rpm. The supernatant was used for various enzyme assays. The catalase (CAT) activity was determined according to Abei [26]. The reaction mixture for measuring CAT activity contained 2.5 ml of phosphate buffer (pH 6.8, 0.1M), 0.5ml of H202(15mM), 0.5ml of enzyme extract. OD was taken at 240nm within 1min of adding enzyme. Guaiacol peroxidase (GPx) activity was measured according to Chance and Maehly [27]. To 2.1ml of phosphate buffer (ph 6.8 ,0.1M), 0.3ml of guaiacol (30mM) , 0.3ml H2O2 (12.3mM),0.3ml of enzyme extract was added. The absorbance was taken at 470nm within 5min of incubation using Biospectrometer Basic, Eppendorf, India. The activity of superoxide dismutase (SOD) was measured by adding 0.2ml enzyme extract to 2.5 ml of phosphate buffer (pH 6.8,0.1M), 0.1 ml BSA (3.3x10-3%),0.1ml riboflavin (0.6mM) and0.1ml NBT (6mM) and taking incubating for 10mins under light. Absorbance was taken at 560nm after 10 min [28]. For measuring gluthathione reductase (GR) activity, to 2ml of 0.2M phosphate buffer (pH 6.8) containing 1mM EDTA, 0.5ML OF 3mM DTNB, 0.1 ml 2mM NADPH, and 0.1 ml enzyme extract was added. Further, 0.2ml of distilled water was added to make the volume upto 2.9ml.then 0.1ml oxidized glutathione (2mM) was added and absorbance was taken at 412nm [29].
Data analysis
Each experiment was conducted three times and data presented are means of three independent repeats ±SE (n=3). LSD test was used for comparision between pairs of treatments. The data analysis was carried out using statistical package, SPSS (ver. 10; SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
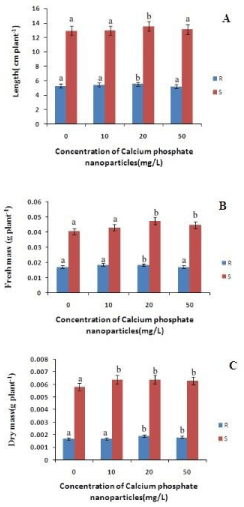
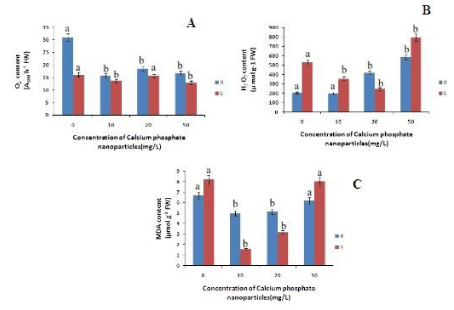
The length of the roots increased with increase in the concentration of calcium phosphate NP with respect to control by 3.4% and 6% at 10mg/L and 20mg/L respectively (Figure 1). However at 50 mg/L lenght decreased by 1% indicating the toxicity of calcium phosphate NP at higher concentration. Similarly the shoot length has also increased with increase in the concentration of calcium phosphate upto 20mg/L by 5% relative to the control. Hence calcium phosphate NP promoted the growth of the rice seedlings as reported in maize by Rane et al., [20]. The root and shoot fresh mass has increased with the concentration of calcium phosphate NP .There is a 8% and 7% increase in root fresh mass compared to control at 10mg/L and 20mg/L treatment of calcium phosphate NP (Figure 1) Similarly in case of shoot fresh mass there is a 8%, 18% and 10% increase at 10mg/L, 20mg/L and 50mg/L respectively. So, it can be concluded calcium phosphate NP increased the fresh mass of the treated rice seedlings (Figure 1). The root dry mass has increased with concomitant increase in calcium phosphate NP concentration. The root dry mass has increased by 11% at 20mg/L calcium phosphate NP treatment. In the case of shoots there is a maximum increase by 10% of shoot dry mass at 10 and 20 mg/L calcium phosphate NP treatment. As depicted in figure 2, the superoxide content in both root and shoot has decreased with increase in concentration of calcium phosphate NP concentration compared to control. In the root the superoxide content has decreased by 49%, 40% and 46% at 10 and 20 mg/L and 50mg/L calcium phosphate NP treatment compared to control. In the shoot there is a maximum decrease of 19.68% at 50mg/L calcium phosphate NP treatment compared to control. The hydrogen peroxide content of root and shoot has decreased compared to control. In case of roots the hydrogen peroxide content has decreased by 2.28% at 10mg/L followed by 105% and 188% increased at 20 mg/L and 50mg/L calcium phosphate NP treatment compared to control. In the shoot the hydrogen peroxide has decreased by 32.8%, 53.6% at 10 and 20 mg/L and increased by 49% at 50 mg/L calcium phosphate NP treatment compared to control (Figure 2). Such finding indicates that calcium phosphate NP at low concentration check ROS generation which may lead to growth promotive function of calcium phosphate NP as reported in the case of maize [20].
Figure 1: Changes in length, fresh mass and dry mass content of rice in root and shoot of rice seedling subjected to control(0 mg/L), 10 mg/L CaPO4 NP, 20 mg/L CaPO4 NP and 50 mg/L CaPO4 NP treatment. Data presented are the mean ± SE (n=3). Letter different from control (0mg/L) over the bar indicates significant mean difference at p<0.05 by LSD test.
The MDA content of both root and shoot has decreased as compared to control. In case of roots the MDA content has decreased by 26%, 23% and 7.2% at 10mg/L, 20 mg/L and 50mg/L calcium phosphate NP treatment compared to control (Figure 2). In shoots there is a decrease in the MDA content by 80%, 60% and 1.9% at 10 and 20 mg/L and 50mg/L calcium phosphate NP treatment compared to control respectively. Decrease lipid peroxidation mediated by calcium phosphate NP may be due to modulation of ROS in plants. As depicted in figure 2, calcium phosphate NP modulates ROS generations in rice seedlings. The level of superoxide anion (O2-) in both root and shoot decreased due to calcium phosphate NP with respect to control (Figure 2).
Figure 2: Changes in superoxide, hydrogen peroxide and MDA content of rice in root and shoot of rice seedling subjected to control(0 mg/L), 10 mg/L CaPO4 NP, 20 mg/L CaPO4 NP and 50 mg/L CaPO4 NP treatment. Data presented are the mean ± SE (n=3). Letter different from control (0mg/L) over the bar indicates significant mean difference at p<0.05 by LSD test.
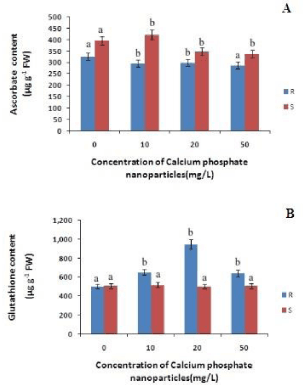
Hydrogen peroxide (H2O2) is the another toxic ROS that may cause cellular damages including lipid peroxidation of membrane. The content of H2O2 in both root and shoot of rice seedlings also decreased only at 10mg/L of calcium phosphate NP relative to control. This indicates that at low concentration of calcium phosphate NP (10mg/L) ROS generations is lowered. The Ascorbate content in roots has decreased with increasing concentration calcium phosphate NP by 9%, 8%, & 11% at 10mg/L 20 mg/L and 50mg/L calcium phosphate NP treatment respectively, as compared to control (Figure 3). Whereas in case of shoots the ascorbate content increased by 6% at 10mg/L concentration of calcium phosphate NP treatment and then decreased by 11%, 14% at 20 mg/L and 50mg/L concentration of calcium phosphate NP treatment relatively to control.
Thus, it indicates that calcium phosphate NP treatment did not significantly modulate ascorbate content. The Glutathione content in root has increased with increasing concentration of calcium phosphate NP by 29%, 88%, and 27% at 10mg/L and 20 mg/L and 50mg/L respectively, as compared to control. In case of shoots the glutathione content has increased by 2% at 10mg/L and slightly decreased at 20 mg/L concentration of calcium phosphate NP treatment but bring no change at 50mg/L concentration of calcium phosphate NP treatment relatively to control (Figure 3).
Figure 3: Changes in total ascorbate and total glutathione content in root and shoot of rice seedling subjected to control(0 mg/L), 10 mg/L CaPO4 NP, 20 mg/L CaPO4 NP and 50 mg/L CaPO4 NP treatment. Data presented are the mean ± SE (n=3). Letter different from control (0mg/L) over the bar indicates significant mean difference at p<0.05 by LSD test.
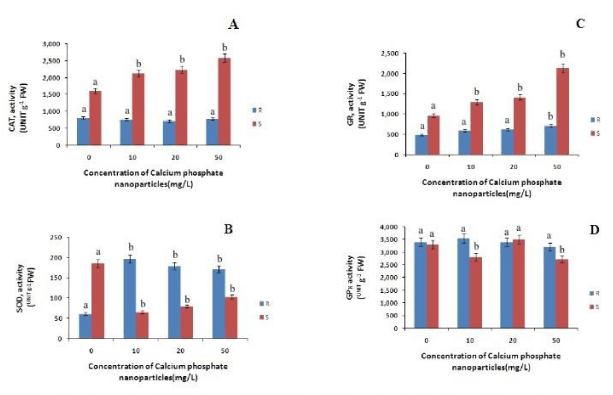
To cope with the rise in the level of ROS accumulation, plants have evolved antioxidant system comprising of enzymes like SOD, CAT, GR, GPX etc., [30]. Superoxide dismutase (SOD) is an important antioxidant enzyme that scavenges the superoxide anion and produces hydrogen peroxide which is then converted to water and oxygen by peroxidase and catalase enzyme.
The CAT content in roots has decreased with increasing concentration and in shoots it is increased with increasing concentration of calcium phosphate NP as compared to control (Figure 4). In case of roots the CAT content is decreased by 6%, 11% and 2% at 10mg/L, 20 mg/L and 50mg/L calcium phosphate NP respectively as compared to control. In case of shoots there is a gradual increase of CAT content by 32%, 38%, and 60% at 10mg/L, 20 mg/L and 50mg/L calcium phosphate NP treatment respectively, relatively to control. The SOD content in root has increased with increasing concentration of calcium phosphate nano-particle. Where as in case of shoot SOD content decreased with increasing concentration of calcium phosphate NP by 64%, 56%,& 44% at 10mg/L and 20 mg/L and 50mg/L calcium phosphate NP treatment respectively as compared to control (Figure 4). The GR content in roots and shoots has increased with the increase in the concentration of the calcium phosphate NP. In case of roots the GR content increased by 22%, 27%, & 47% at 10mg/L 20 mg/L and 50mg/L calcium phosphate NP treatment respectively, as compared to control. Where as in case of shoots the GR content increased by 34%, 45% & 100% at 10mg/L, 20 mg/L and 50mg/L calcium phosphate NP treatment respectively relative to control. The GPx content in roots has increased with the increase in the concentration of the calcium phosphate NP. In case of roots the GPx content increased by 4.4%, 0.39% at 10mg/L and 20 mg/L and decreased by 5.3% at 50mg/L calcium phosphate NP treatment respectively, as compared to control (Figure 4). Whereas in case of shoots the GR content increased by 25% at 20 mg/L calcium phosphate NP treatment relative to control. Interestingly GPx activity has decreased in the shoots at 10mg/L and 50mg/L calcium phosphate NP treatment respectively in comparison to control.
Figure 4: Changes in catalase (CAT), gluthathione reductase (GR), superoxide dismutase (SOD) and guaiacol peroxidase( GPx) activities in root and shoot of rice seedling subjected to control(0 mg/L), 10 mg/L CaPO4NP, 20 mg/L CaPO4NP and 50 mg/L CaPO4NP treatment. Data presented are the mean ± SE (n=3). Letter different from control (0mg/L) over the bar indicates significant mean difference at p<0.05 by LSD test.
Thus, calcium phosphate nanoparticles have shown to promote growth of shoot and root in rice plants. It modulates ROS and antioxidant responses by stimulating antioxidant system including enzymes and metabolites.
CONCLUSION
Ca3(PO4)2 NP alters growth and antioxidant responses in dose dependent manner. Ca3(PO4)2 NP improves growth and lower ROS at 10 & 20 mg/L concentration. Thus, Ca3(PO4)2 NP may interect with plant and alter physiological changes depending on dose. Calcium phosphate nanoparticles may help in formulation of new nano growth promoter and nanofertilizers for agricultural use. Therefore, it could potentially help in reduction of the quantity of fertilizer applied to crops and contributing to precision farming as it reduces fertilizer wastage and in turn environmental pollution due to agricultural malpractices. However, detail physiological and molecular understanding of its impact on rice or other crop plant is needed in future to validate its prospective application in agriculture.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests regarding the publication of this paper.
ACKNOWLEDGEMENT
The author express his sincere thanks to the DBT, Govt of India, for funding IBH facility to Karimganj College Karimganj, Assam, India. I am thankful to Dr M K Bhattacharya, Coordinator IBH Karimganj College, Karimganj, Assam, India for allowing Laboratory facilities to be used during the work. I, further extend my gratitude to Dr B P Baruah, Chief Scientist, RARS, Akbarpur, Karimganj for supplying rice seeds throughout the experiment.
REFERENCES
- Siddiqui MH, Al-Whaibi MH. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J Biol Sci. 2014; 21: 13-17. Ref.: https://goo.gl/XDAqGr
- Jampílek J, Kráľová K. Application of nanotechnology in agriculture and food industry, its prospects and risks. Ecological Chemistry and Engineering Sci. 2015; 22: 321-361. Ref.: https://goo.gl/RlnGP0
- Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nature materials. 2014; 13: 400-408. Ref.: https://goo.gl/9Y7ois
- Mahajan S, Pandey GK, Tuteja N. Calcium-and salt-stress signaling in plants: shedding light on SOS pathway. Arch Biochem Biophys. 2008; 471: 146-158. Ref.: https://goo.gl/McGh5Z
- Lutts S, Kinet JM, Bouharmont J. Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance”. Plant Growth Regulation. 1996; 19: 207-218. Ref.: https://goo.gl/jPVuph
- Knight H. Calcium signaling during abiotic stress in plants. Int Rev Cytol. 1999; 195: 269-324. Ref.: https://goo.gl/uekn95
- Asano T, Hayashi N, Kobayashi M, Aoki N, Miyao A et al. A rice calcium‐dependent protein kinase OsCPK12 oppositely modulates salt‐stress tolerance and blast disease resistance. Plant J. 2012. 69: 26-36. Ref.: https://goo.gl/fSrGNV
- Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007; 581: 2325-2336. Ref.: https://goo.gl/1rabbB
- Das R, Pandey GK. Expressional Analysis and Role of Calcium Regulated Kinases in Abiotic Stress Signaling. Curr Genomics. 2014; 11: 2-13. Ref.: https://goo.gl/zDMq0l
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002; 14: S401-S417. Ref.: https://goo.gl/JSjWRv
- Trewavas AJ, Malhó R. Ca2+ signaling in plant cells: the big network. Curr Opin Plant Biol. 1998; 1: 428-433. Ref.: https://goo.gl/YZnYQ0
- McAinsh MR, Gray JE, Hetherington AM, Leckie CP, Ng C. Ca2+ signalling in stomatal guard cells. Biochem Soc Trans. 2000; 28: 476-481. Ref.: https://goo.gl/cjGBrI
- Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell.2002; 14: S389-S400. Ref.: https://goo.gl/QwNHa1
- Snedden WA, Fromm H. Calmodulin as a versatile calcium signal transducer in plants. New Phytologist. 2001; 151: 35-66. Ref.: https://goo.gl/lrsOUx
- Zheng L, Hong F, Lu S, Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res. 2005; 106: 279-297. Ref.: https://goo.gl/2hkbv6
- Galbraith DW. Nanobiotechnology: silica breaks through in plants. Nat Nanotechnol. 2007; 2: 272-273. Ref.: https://goo.gl/nTbR5o
- Mahajan P, Dhoke SK, Khanna AS. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna radiata) and Gram(Cicer arietinum) Seedlings Using Plant Agar Method. Journal of Nanotechnology. 2011; 2011: 1- 7. Ref.: https://goo.gl/gCskOk
- Boonyanitipong P, Kositsup B, Kumar P, Baruah S, Dutta J. Toxicity of ZnO and TiO2 Nanoparticles on Germinating Rice Seed Oryza sativa L. International Journal of Bioscience, Biochemistry Bioinformatics. 2011; 1: 282-285. Ref.: https://goo.gl/BNxhy2
- Liu R, Zhang H, Lal R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients? Water, Air, and Soil Pollution. 2016; 227: 1-14. Ref.: https://goo.gl/0LWVpR
- Rane M, Bawskar M, Rathod D, Nagaonkar D, Rai M. Influence of calcium phosphate nanoparticles, Piriformospora indica and Glomus mosseae on growth of Zea mays. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2015; 6: 045014. Ref.: https://goo.gl/X8XyRI
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatable race of Phytophthora infestans and to the hyphal wall components. Physiological Plant Pathology. 1983; 23: 345-357. Ref.: https://goo.gl/cylbDi
- Lin CC, Kao CH. Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant and Soil. 2001; 230: 135-143. Ref.: https://goo.gl/R1Qa1g
- Zhang XZ. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In Research Methodology of Crop physiology; Zhang, X.Z., Ed.; Agricultural Press: Beijing, China. 1992; 208-254.
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980; 106: 207-212. Ref.: https://goo.gl/mhMJT6
- Oser BL. Hawks physiological chemistry. McGraw Hill, NY, USA. 1979; 702-705.
- Abei H. Catalse in vitro. Methods Enzymol. 1984; 105: 121-126. Ref.: https://goo.gl/EK2zJg
- Chance B, Maehly AC. Assay of catalase and peroxidises. Methods Enzymology. 1955; 2: 764-775. Ref.: https://goo.gl/noFgcH
- Giannopolitis CN, Ries SK. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1997; 59: 309-314. Ref.: https://goo.gl/fVDTTx
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5, 51-dithiobis(2-nitrobenzoic acid). Anal Biochem. 1988; 175: 408-413. Ref.: https://goo.gl/EumbPn
- Upadhyaya H, Dutta BK, Panda SK. Zinc modulates drought induced biochemical damages in tea [Camellia sinensis (L) O Kuntze]. J Agric Food Chem. 2013; 61: 6660-6670. Ref.: https://goo.gl/979vsz