More Information
Submitted: 16 October 2019 | Approved: 30 October 2019 | Published: 31 October 2019
How to cite this article: Brandao-Costa RMP, Batistaa JMS, Nascimento TP, Porto ALF. Renal function effects of FDS, a saponin isolated from Filicium decipiens seeds: Biochemical and Histopathological studies. J Plant Sci Phytopathol. 2019; 3: 107-110.
DOI: 10.29328/journal.jpsp.1001040
Copyright License: © 2019 Brandao-Costa RMP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nephrotoxicity; Renal failure; Saponin; Biological effect; Biochemical analysis
Renal function effects of FDS, a saponin isolated from Filicium decipiens seeds: Biochemical and Histopathological studies
Romero MP Brandao-Costa*, Juanize Matias S Batistaa, Thiago Pajeu Nascimento and Ana LF Porto
Department of Morphology and Animal Physiology, Laboratory of Bioactive Products and Technology, Federal Rural University of Pernambuco - UFRPE, Brazil
*Address for Correspondence: Romero Brandao-Costa, Department of Morphology and Animal Physiology, Laboratory of Bioactive Products and Technology, Federal Rural University of Pernambuco - UFRPE, Dom Manoel de Medeiros Street, s/n, Dois Irmãos - CEP: 52171-900 - Recife/PE, Brazil, Tel: +55.81.3320.6345; Fax: +55.81.3320.6345; Email: romero_brandao@outlook.pt; romero_brandao@yahoo.com.br
Physicochemical and pharmacological studies indicated that Filicium decipiens seeds contained various specialized metabolites, including saponins. The aim of this work is to reveal the nephrotoxicity of FDS, a saponin isolated from Filicium decipiens seeds on male Wistar rats histopathological and biochemical parameters. Rats were submitted to oral ingestion of FDS (6.0 mg/kg) and crude extract (120.0 mg/kg) and were observed high levels of urea and creatinine in blood analyses of all animals followed by an acute renal failure by glomerular retraction. In the present study, FDS and crude extract when administered in Wistar rats induced an increase of serum levels of Urea and Creatinine, biochemical markers of kidney function. Table 1 shows Urea concentration at Test group with FDS (54.3 ± 1.80 mg/ml) and Test group with crude extract (49.7 ± 2.00 mg/ml), were 47% and 34.7% higher, respectively, when compared to control group (36.9 ± 2.00 mg/ml), and Creatinine at the test group with FDS (2.1 ± 0.03 mg/ml) and test group with crude extract (1.6 ± 0.09 mg/ml) presented a value 3.5 and 2.8 times higher, respectively, than control (0.6 ± 0.08 mg/ml). Based on these results, our data demonstrate a significant effect in renal function of rats treated with F. decipiens saponin.
Saponins are a group of surfactants of natural origin belonging to the glycoside derivatives of steroids or polycyclic triterpenes [1]. They are found as secondary metabolites in many species of plants and some species of marine animals and insects. Global saponin market is very promising, have a forecast for this growth annual of roughly 0.2% over the next five years, will reach $ 970 million in 2023.
There are several uses of saponins, being applied in industries of food, cosmetics, additives to animal feed and for plant protection in agriculture. Literature reported that saponins may have anti-inflammatory, anti-tumor, anti-hyperuricemia, immunomodulatory, lipid-regulating and may exhibits other pharmacological activities, such as neuroprotective and renoprotective effects [2]. Saponin-based adjuvants are promising adjuvants that enhance both humoral and T-cell-mediated immunity. One of the most used natural products as vaccine adjuvants are Quillaja saponaria bark saponins and its fraction named Quil A.
Therefore, steroidal saponins have attracted attention as virtually untouched stuff with potential to produce lots of steroidal hormones as well as promising pharmaceutically effective compounds [3]. Generally, these compounds are formed from a hydrophobic aglycone, called sapogenin, linked to one or more hydrophilic sugar moieties through an ether or ester glycosidic linkage, at an or two glycosylation sites. The aglycone part, also called sapogenin, is either a steroid (C27) or a triterpene (C30). Steroidal saponins are sugar conjugates of C27 steroidal compounds, in which the side chains are modified to form a spirochetal (spirostane saponins) or a hemiketal (furostane saponins) [4-6].
An example of a plant that can be used as a source of saponin is Filicium decipiens (Wright and Am.) Thwaites is a Large tree usually found in southern Africa. This species has attractive, bright fern-like foliage and a free translation of your botanical name is misleading fern type. This plant is already used by some people to treat diabetes, is also promising in bioactive compounds, the literature also reports synthesis of palladium nanoparticles using Filicium decipiens leaf extract [7-11]. Some studies have already shown the extraction of saponin of Filicium decipiens demonstrating a variety of biological activities, e.g. antifungal, antibacterial and molluscicidal activities, despite presenting high toxicity [12].
In the last decades the interest in natural treatments in general and herbal medicine has increasingly attracted its use. However natural remedies based on plants are not harmless may even be windy. In this sense, the objective of this work was to evaluate the renal function effect of FDS, a saponin extracted from Filicium decipiens seeds.
Chemicals
All other solvents/chemicals used were of analytical grade.
Extraction and Isolation of FDS, a saponin previously purified from F. decipiens seeds
F. decipiens (Wight & Arn.) seeds were harvested from the top of the plant at the Federal University of Pernambuco campus in Brazil. The botanical specimen was identified at the DÁRDANO DE ANDRADE LIMA Herbarium of the Institute of Agricultural Research, where a voucher was deposited [Botanical identification Nº 64/2006; Identification code IPA-88.039; F. decipiens (Wight & Arn.)]. The common name of this plant in Brazil is Cabaceira. The seeds were briefly washed with distilled water and left to dry at 25°C for 10 days. Dried seeds (10 g) were then powdered, homogenized in 10% (w/v) 0.15 mol/L NaCl and maintained under agitation at room temperature for 30 min. Afterwards, the mixture was filtered through gauze and centrifuged at 11,180 x g for 15 min. The supernatant, termed the crude extract, was then studied using chromatographic methods. All the procedures to obtain FDS were conducted according to Brandao-Costa, et al. [2]. Briefly, The sample obtained from crude extract was roughly chromatographed (1.0 ml at a time) on a Sephadex LH-20 column (6.5 × 50 cm) and eluted with 200 ml MeOH (25 ml fractions) to isolate saponin. At all times, the elution absorbance was monitored at 254 nm on a Spectrophotometer 3000 (Bio-Rad).
Animals and experimental treatment
Ethics statements: All experimental procedures involving the animals were approved by the Committee for Ethics in Animal Experimentation of the Federal University of Pernambuco (No. 23076.012671/2006-09). All animal procedures were in accordance with the Brazilian College of Animal Experimentation – COBEA and approved by The Animal Ethics Committee of the Federal University of Pernambuco.
Male Wistar rats (n = 24), aged 13 weeks and weighing 340.5 ± 5 g, were used. They were raised and housed two to a cage at a vivarium of the Anatomy Department of UFPE, under controlled environmental conditions (photoperiod of 12h light/dark, temperature of 23 ± 1°C). The animals were divided into three groups: negative control (n = 4), test group (n = 10) using FDS dissolved in water and second test group (n = 10) using Crude extract dissolved in water. The groups were fed standard rations ad libitum. The test group (T1) was submitted to oral ingestion of saponin FDS (6.0 mg/kg solution, 1ml) twice a day for 10 days times and the test group (T2) was submitted to oral ingestion of crude extract (120.0 mg/kg solution, 1 ml) twice a day for 10 days times too. The drugs were diluted in purified water to a final concentration of 2.043 mg/ml and 40.86 mg/ml for FDS and crude extract, respectively, and 1.0 ml was administered in each animal. After the treatment period, the rats were subcutaneously anesthetized using two anesthetic-sedative drugs: 0.02 ml (23.0 mg/ml) xylazine chloride and 0.07 ml (50.0 mg/ml) ketamine chloride, for each 40 g of body weight. Blood samples were extracted through intracardiac puncture and then the animals were euthanized through left ventricular perfusion with a fixing solution of 4% glutaraldehyde in 0.05 M phosphate buffer, pH 7.4. Subsequently, their kidneys were removed.
Serum biochemical analysis
Blood samples for biochemical analyses were obtained by intracardiac puncture at the end of the experiment and placed into coagulant micro-blood tubes of 500 µL capacity. After 15 min, the clotted blood was centrifuged (KUBOTA KR20000T) at 3,000 g, for 15 min, at 25°C. The serum was separated and used to evaluate urea (Urease GLDH Diasys), creatinine (Architect Abbot), alanine aminotransferase and aspartate aminotransferase (UV Diasys-Architect c8000), alkaline phosphatase (p-NPP Diasys), by an automated random-access clinical chemistry analyzer (Abbott Aerosets).
Histopathological studies
Kidney histological analyses: To evaluate the effects of saponin and extract bioactive compounds on renal function of rats, a histological study was conducted according to Saberi, et al. [13]. Briefly, the kidneys from the normal and experimental mice were kept in cold isotonic buffer (250.0 mM sucrose, 10.0 mM HEPES-Tris (pH 7.4), 2.0 mM EDTA and 0.15 mg/ml trypsin inhibitor (type II-S) supplemented with 1.0 mM PMSF). Afterward, kidneys were fixed in 10% buffered formalin and processed for paraffin sectioning. Thin (0.5 mm) transverse slices of the cortex corticis were removed with a Stadie–Riggs microtome and carefully dissected with small scissors to eliminate contamination from the rest of the tissue. Subsequently, sections were stained with hematoxylin and eosin to evaluate under a light microscope.
Statistical analysis
One-way analysis of variance (ANOVA) was applied using STATISTICA for Windows (version 6.0, Statsoft Inc., USA). The data obtained were expressed as means ± standard error of mean (SEM). Origin 6.0 software was used to plot the data.
F. decipiens is a plant largely found in Brazil, South Africa and the northeast of India. F. decipiens seeds were collected and submitted to extraction with 0.15 mol/L NaCl (saline solution) to obtain a crude extract. A purification process using a coupled ion-exchange system with DEAE-Sephadex and CM-Sephadex resins, followed by Sephadex LH-20 was used to obtain FDS.
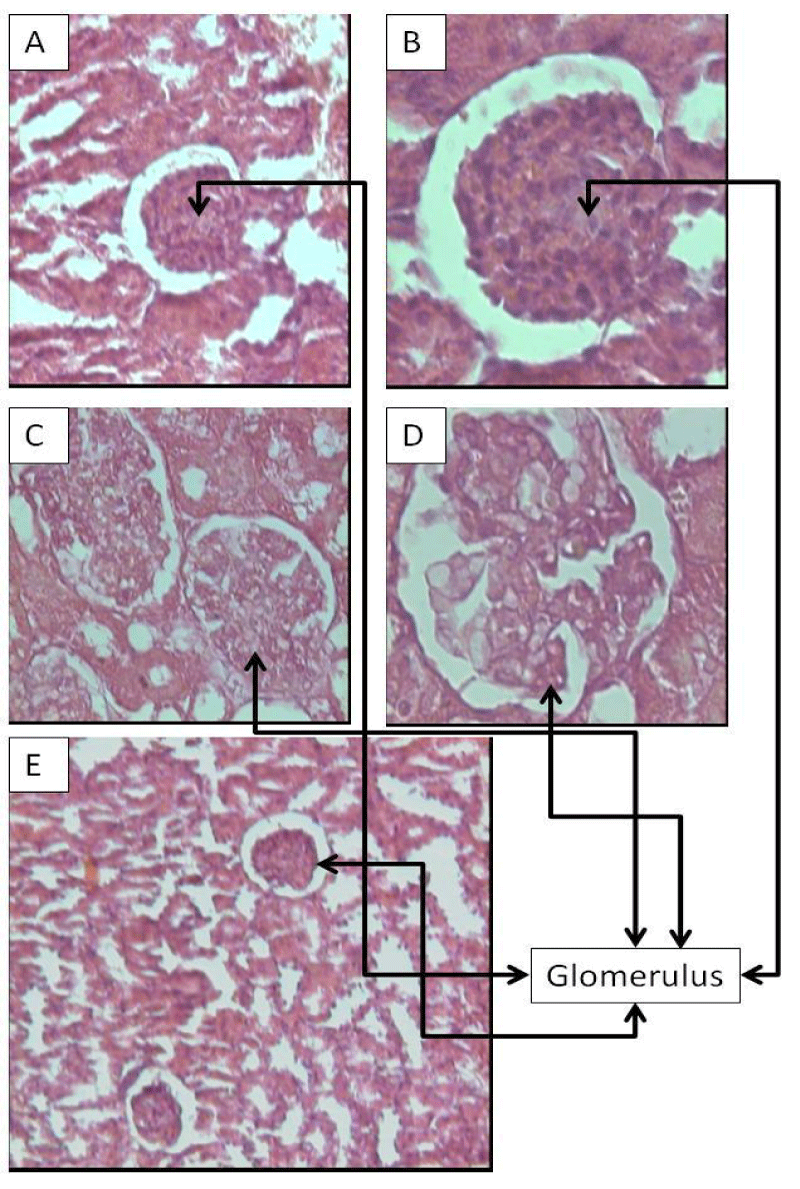
In the present study, FDS and crude extract when administered in Wistar rats induced an increase of serum levels of Urea and Creatinine, biochemical markers of kidney function. Table 1 shows Urea concentration at Test group with FDS (54.3 ± 1.80 mg/ml) and Test group with crude extract (49.7 ± 2.00 mg/ml), were 47% and 34.7% higher, respectively, when compared to control group (36.9 ± 2.00 mg/ml), and Creatinine at the test group with FDS (2.1 ± 0.03 mg/ml) and test group with crude extract (1.6 ± 0.09 mg/ml) presented a value 3.5 and 2.8 times higher, respectively, than control (0.6 ± 0.08 mg/ml). Still in table 1, we observed that the crude extract induced a hepatocellular injury because the alanine aminotransferase and aspartate aminotransferase activities were significantly increasing. Different results were achieved with FDS, which suggests crude extract contains other active molecules. After obtaining these results, the kidneys of the animals were histopathological analyzed aiming to investigate the possible effects of FDS, and it was observed that there was really a retraction on glomerular structures of rats treated (Figure 1A,B), when compared to control group (Figure 1C,D), thus justifying the increase of the levels of biomarkers of renal function. The present study has evaluated the effect of FDS on renal function and to confirm the evidence of renal failure caused by FDS, crude extract was also submitted to oral ingestion and was detected really the effect of FDS. Figure 1E shows a section of kidney of rats treated with crude extract presenting the same retraction on glomerular structures caused by FDS. This suggests that renal failure induced by crude extract was exactly caused by FDS. According Weng [14], histopathological examinations of kidneys of rats treated with ginsenosides inhibited the pathological changes such as mild and moderate glomerular tuft retraction to acute tubular cell degeneration and tubular swelling induced by cantharidin. In all, ginsenosides have protective activity on acute renal injury induced by cantharidin. Wisløff, et al. [15] reported in their experiment using Narthecium ossifragum leaf extract, during the first 11 days of the experiment, six rats were found dead or had to be euthanized due to dominant signs, diarrhea and dehydration. Authors also reported that N. ossifragum leaf extract causes acute nephrotoxicity or renal damage in several ruminant species.
| Table 1: Effect of FDS and Crude extract on rat biochemical parameters. | |||
| Parameters | Controla | FDSa,b | Crude extracta,c |
| Aspartate aminotransferase (IU L-1) | 122.6 ± 7.70 | 127.3 ± 2.10 | 172.8 ± 4.10c |
| Alanine aminotransferase (IU L-1) | 38.8 ± 6.10 | 36.3 ± 2.10 | 68.8 ± 8.10c |
| Alkaline phosphatase (IU L-1) | 228.0 ± 2.28 | 224.0 ± 4.20 | 236.0 ± 3.18c |
| Urea (mg mL-1) | 36.9 ± 2.00 | 54.3 ± 1.80b | 49.7 ± 2.00c |
| Creatinine (mg mL-1) | 0.6 ± 0.08 | 2.1 ± 0.03b | 1.6 ± 0.09c |
| aValues are represented as mean ± SD (n = 6). bp < 0.05 compared with control group; Student t - test was used to assess the statistical significance. cp < 0.05 compared with control group; Student t - test was used to assess the statistical significance. |
|||
Figure 1: Histopathological examination of renal injured tissue of rat. Hematoxylin and eosin stained (magnification, 20 - 40x•): (A, magnification, 20x) and (B, magnification, 40x) The test group treated with FDS (6.0 mg/kg) showed glomerular tuft retraction (solid arrows) and cloudy swelling of tubules. (C, magnification, 20x) and (D, magnification, 40x) The normal control group, showed normal glomerulus and tubles; (E, magnification, 10x) The test group treated with crude extract (120.0 mg/kg) showed glomerular tuft retraction (solid arrows) and cloudy swelling of tubules.
Literature has been discussed whether traces of saponins could be absorbed through the permeabilized membranes, but all evidence until now has pointed to their non-absorption. The findings in the present study, however, clearly show that saponins can be absorbed from the gastrointestinal tract [16]. Saponins are usually not associated with renal damage, but when absorbed, their membrane-permeabilizing effect may possibly be detrimental to the renal epithelial cells. The kidneys are very sensitive to the action of toxic agents. One reason for this is the fact that the kidneys, although comprising only 0.4% of the body weight, receive 25% of the resting cardiac output, which means that the kidney is exposed excessively to toxic agents present in the circulation [17].
Another reason is that the nephron, with its ability to concentrate solutes filtered by the glomeruli, may be exposed to very high concentrations of toxic agents in some parts of its course. The tubules are particularly sensitive to toxic influences, in part because they have a high oxygen consumption and vulnerable enzyme systems, and in part because they have complicated transport mechanisms that may be used for transport of toxins or may be damaged by them. The histopathological renal changes observed in the present study had no similarities to observations in other experiments.
Medicinal plants have always had a special place in rural communities. The increasing trend of medicinal plants uses for traditional medicine is due to the general lack of access to modern health care facilities for rural communities. FDS demonstrated several biological implications mainly when tested through in vivo study, crude extract and FDS showed nephrotoxicity in rats. These new results suggest that FDS can induce renal failure and cause kidney damage and may be dangerous for Traditional uses.
However, further study needs to be conducted focusing on its efficacy in clinical application for other pharmacological features.
This research was financially supported by research grants and fellowships from the Fundação de Amparo a Ciência e Tecnologia (FACEPE). The authors also express their gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
- Rekiel E, Smułek W, Zdziennicka A, Kaczorek E, Jańczuk B, et al. Wetting properties of Saponaria officinalis saponins. Colloid Surf A Physicochem Eng Asp. 2019; 584: 123980.
- Brandão-Costa RMP, Nascimento TP, Bezerra RP, Porto ALF. FDS. A novel saponin isolated from Felicium decipiens: Lectin interaction and biological complementary activities. Process Biochemistry. 2019.
- Tamura Y, Miyakoshi M, Yamamoto M. Application of saponin-containing plants in foods and cosmetics. In: Hiroshi Sakagami, Textbook of Alternative Medicine. 2012; 83-101.
- Kayukawa CTM, Oliveira MAS, Kaspchak E, Sanchuki HBS, Igarashi-Mafra L, et al. Quillaja bark saponin effects on Kluyveromyces lactis-galactosidase activity and structure. Food Chem. 2020; 15: 303-125388. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31454757
- Lavaud C, Voutqkwne L, Massiot G, Le Men-Olivier L, Das BC, et al. Saponins from the stem bark of Filicium decipiens. Phyfochemistry. 1998; 47: 441-449. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9433818
- Coates Palgrave K, Drummond RB, Moll JE, Coates PM. 3rd ed. Trees of Southern Africa. Struik Publishers Cape Town. 1984; 541.
- Jayasinghea UL, Bandara N, Fujimoto Y. A new norneohopane caffeate from Filicium decipiens. Fitoterapia. 2001; 72: 737-742. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11677010
- Sharmila G, Fathima MF, Haries S, Geetha S, Kumar MN, et al. Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J Molecular Structure. 2017; 1138: 35-40.
- Sasikala C, Yajaman S. Effect of compounds isolated from Filicium decipiens and Ventilago madraspatana against diabetic nephropathy in streptozotocin induced diabetic rats. Indian J Pharmaceutical Education Res. 2015; 49.
- Akila E, Geetha Priya C. Phytochemical screening and antidiabetic, antihyperlipidemic properties of leaves of Filicium decipiens in streptozotocin induced diabetic rats. J Drug Delivery & Therapeutics. 2019; 9: 62-66.
- Man S, Gao W, Zhang Y, Huang L, Liu Ch, et al. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010; 81: 703-714. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20550961
- Mahyuni S, Sofihidayati T. KADAR saponin dan aktivitas antibakteri ekstrak daun Filicium decipiens (Wight & Arn.) Thwaites TERHADAP Staphylococcus aureus, Escherichia coli dan Candida albicans. FITOFARMAKA J Ilmiah Farmasi. 2018; 8.
- Saberi H, Keshavarzi B, Shirpoor A, Gharalari FH, Rasmi Y. Rescue effects of ginger extract on dose dependent radiation-induced histological and biochemical changes in the kidneys of male Wistar rats Biomedicine & Pharmacotherapy. 2017; 94: 569-576 .
- Wisløff H, Flåøyen A, Ottesen N, Hovig T. Narthecium ossifragum (L.) huds. causes kidney damage in goats: morphologic and functional effects. Vet Pathol. 2003; 40: 317-27. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12724574
- Konda VR, Arunachalam R, Eerike M, Rao KR, Radhakrishnan AK, et al. Nephroprotective effect of ethanolic extract of Azima tetracantha root in glycerol induced acute renal failure in Wistar albino rats. J Tradit Complement Med. 2015; 26: 347-354. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27774418
- Saha S, Sadhukhan P, Sinha K, Agarwal N, Si PC. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. BB Reports. 2016; 5: 313-327. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28955838
- Sahu BD, Tatireddy S, Koneru M, Borkar RM, Kumar JM, et al. Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats. Possible mechanism of nephroprotection. Toxicol Appl Pharmacol. 2014; 15: 8-20. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24637089