More Information
Submitted: December 29, 2022 | Approved: January 05, 2023 | Published: January 06, 2023
How to cite this article: Chen D, Hu QJ, Chen XF. Control of rice bakanae disease by seed dressing with mixed solution of fludioxonil, metalaxyl-M and azoxystrobin. J Plant Sci Phytopathol. 2023; 7: 001-007.
DOI: 10.29328/journal.jpsp.1001096
Copyright License: © 2023 Chen D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Rice bakanae disease; Fludioxonil; Metalaxyl-M; Azoxystrobin; Seed dressing; Fusarium fujikuroi
Control of rice bakanae disease by seed dressing with mixed solution of fludioxonil, metalaxyl-M and azoxystrobin
Deng Chen1,2, Qi-Juan Hu1 and Xiao-Feng Chen1*
1Guangdong Provincial Enterprise Key Laboratory of Seed and Seedling Health Management Technology, Shenzhen Noposion Agrochemical Co., Ltd, Shenzhen, Guangdong Province, China
2College of Plant Protection, South China Agricultural University, Guangzhou 510642, Guangdong Province, China
*Address for Correspondence: Xiao-Feng Chen, Guangdong Provincial Enterprise Key Laboratory of Seed and Seedling Health Management Technology, Shenzhen Noposion Agrochemical Co., Ltd, Shenzhen, Guangdong Province, China, Email: 283285666@qq.com
Rice bakanae disease is a typical seed-borne disease caused by Fusarium fujikuroi that occurs in seedling beds and in fields. Fungicide seed treatment is an effective solution to this disease. In this study, we used a triple-fungicide suspension identified as 11% FMA, which is composed of 1.1% fludioxonil, 3.3% metalaxyl-M and 6.6% azoxystrobin to coat rice seeds for the prevention of bakanae disease. 11% FMA is water-logging resistant for rice seed treatment. Results showed that the mycelial growth of F. fujikuroi was significantly inhibited by 11% FMA in the laboratory test. Seed dressing with FMA at the rate of 1, 2, and 4 g per kg of seeds promoted seed germination and growth of seedling roots. Treatment with 11% FMA under all dose rates prevented rice bakanae disease of seedlings by more than 90%, especially by more than 95% at 4, 6 and 8 g per kg of seeds. During the subsequent maturation period, rice bakanae control efficiency reaches above 95% as well at 6 or 8 g per kg of seeds, slightly larger than about 92% at 1 or 2 g per kg of seeds. Above all, the rice yield notably improved by 11% with 1 g/kg, by around 8% with 2, 4, or 8 g/kg and by 5% with 6 g/kg treatment.
Rice bakanae is one of the noteworthy seed-borne diseases in many rice-planting areas [1-4]. The causal agent of this disease is the filamentous genus Fusarium, which is mostly composed of F. verticillioides, F. fujikuroi and F. proliferatum [5-7]. Rice plants can be infected and damaged by this disease from the seedling to the maturation stages. Rice seeds severely contaminated with the fungus result in poor germination or withering [7]. Plants infected with this disease exhibited elongated and slender stems that led to the formation of thin, chlorotic leaves and abnormal branches. Severely affected seedlings usually died shortly after sowing [2,8,9]. Slightly infected seedlings transplanted to the field showed crown rot or entropy until the tillering stage, resulting in poorly filled or empty grains [2,5,8-10]. Yield reduction caused by rice bakanae disease can reach 10% - 20%, and under severe conditions, production losses may reach 50% [11].
Using fungicides to treat seeds has been a management tool to prevent rice bakanae. Benzimidazole fungicides (mainly carbendazim) have been widely used to control this disease since the 1980s [10,12,13]. In the last decade, fluazinam, prochloraz and thiram were popular to use against bakanae [7,14-16]. However, because of the continuous use of these chemicals at high concentrations, resistance in F. fujikuroi has occurred in recent years and resulting in control failures in many countries [12,17]. Based on this fact, it was necessary to develop novel chemical mixes or application methods to treat seeds. In past years, many active chemicals have been developed for seed treatments to fight against fungal pathogens. Recently, a novel cyanoacrylate fungicide, phenamacril, developed and patented by Jiangsu Pesticide Research Institute Co., Ltd., has been used through seed coating or soaking [13,18]. Seed coating is an advanced technique with better effect and lower toxicity allowing, effective disease control and judicious use of pesticides [7].
Among many fungicides, fludioxonil, metalaxyl-M, and azoxystrobin are effective on many seedling pathogens. Fludioxonil, FRAC (Fungicide Resistance Action Committee) code 12 [19], was synthesized and developed by Novartis (Basel, Switzerland). Fludioxonil has low toxicity and is effective against many genera of fungi, including Aspergillus, Ascochyta, and Fusarium [14], resulting in broad application possibilities and a promising market. Fludioxonil was used for seed treatment by Syngenta in Basel, Switzerland [7] and in China, and was registered as a seed- coating fungicide for cereals, corn, cotton, and other crops. Metalaxyl-M, works by interfering with nucleic acid metabolism and was assigned to FRAC group code 4. It is specifically effective against diseases caused by oomycetes [20-23]. The material is mostly used for seed or soil treatment and to protect crop seeds from underground pathogens. Azoxystrobin, a broad- spectrum methoxy acrylate pesticide used as a foliar spray and seed treatment, by disturbing respiration in mitochondria [24-26].
Fungicides with a single mode of action have a high risk of selecting for resistance in pathogen populations [27]. In recent years, it has been common to mix two or three modes -of -action into a single product to mitigate this risk. Pre-mix with multiple MOA may act in a synergistic or complementary manner to reduce fungicide resistance and protect seedlings simultaneously [5,27]. In this study, the mixtures of fludioxonil, metalaxyl-M and azoxystrobin were prepared as a suspended seed dressing concentrate used to coat rice seeds at a series of doses. By investigating the plant during the whole growth period, we explored the effect on seedling growth and disease control.
Fungicides, isolates and media
Based upon previous formulation screening and bioassays, the ratio (w/w/w) of fludioxonil to metalaxyl-M to azoxystrobin in the mixture was set as 1:3:6 (data not published). The concentration of fludioxonil, metalaxyl-M, and azoxystrobin was 1.1%, 3.3% and 6.6%, respectively. For this publication, the fungicide mixture was named 11% FMA based on the first letter of the respective chemicals. The trade name of the product was Runmiao, which means nourishing seedlings in Chinese. It was provided by the research laboratory of Shenzhen Noposion Agrochemical Co., Ltd.
PDA culture medium was prepared with 200 g of potato, 20 g of dextrose and 18 g of agar (Sigma) per liter of distilled water.
The Fusarium fujikuroi strain was isolated from the field and identified by Nanjing Agricultural University. Mycelial plugs (5 mm diameter) were collected from 5-day-old colonies and transferred to the center of a plastic petri dish containing PDA (potato dextrose agar) culture medium. These plates were cultured in darkness for 72 h at 28 °C in an incubator.
For a single spore separation assay, Water Agar (WA) was made with 16 g of agar (Sigma) per liter of distilled water. Both PDA and WA media were autoclaved at 121 °C for 20 min before use.
Measurement of colony size
Different dilution ratios of FMA were prepared to add to the PDA medium with mixing completely, followed by pouring into the binary petri dish. Mycelial plugs of 5 mm diameter were transferred to the center of PDA plates. Colony sizes with three replicates were measured after culturing for 72 h at 28 °C.
Seed treatment laboratory assay
For coating assay, 11% FMA at the doses of 1 g/kg (FMA: seed = 1:1000, w/w), 2 g/kg (1:500), 4 g/kg (1:250), 6 g/kg (1:167) and 8 g/kg (1:125) were respectively used to coat rice seeds by mixing with according amounts of distilled water. The ratio (w/w) of the chemical solution to the rice seeds was set at 1:50. The cultivar of the rice seed was Longjing31, a japonica variety bred by Rice Research Institute, Heilongjiang Academy of Agricultural Sciences of China. The chemical solution and rice seeds were both put into a bag for coating. The bag was sealed and thoroughly shaken manually for 2 min. After that, all seeds were evenly colored red. The coated seeds were then spread on a copy paper to aerate and loosen lumps for 10 min before storing or sowing.
For the water-soaking resistance experiment, 10 g of coated rice seeds were put into a conical flask containing half a volume of distilled water. Thirty minutes later seeds were taken out of the flask with a sieve. The appearance of coated seeds and remaining water were observed.
Germination experiment
For the seed germination assay, the anchor seed germination paper (Code: SD3836S, Anchor Paper Company, USA) was soaked in distilled water for 5 min, followed by spreading 100 rice seeds evenly onto it [28]. The untreated seeds were used as the control. The paper was carefully rolled up and put into the greenhouse at a temperature of 26 °C and a relative humidity of 60%. The light regime was 12 h lightness and 12 h darkness in circulation. After incubating for 3 days, the anchor seed germination paper was taken out and unfolded to detect germination. Seeds were considered germinated when the germinal length was longer than that of the seed. The total germination rate was calculated by the ratio of germinated seeds number to whole seeds number.
Seed treatment with fungal inoculation
To increase the possibility of the rice bakanae disease happening, rice seeds were exposed to a fungal solution before being treated with the chemical. The fusarium fujikuroi strain was cultured on the PDA medium for 7 days at 28°C in darkness. Three plates of hyphae were disturbed with a glass rod carefully and transferred into 2 L of PDB medium. The fungal solution was incubated in a shaker at 160 r/min and 28 °C for 24 h in darkness. After that, the conidial solution diluted to 106 spores per milliliter was detected by a blood counting chamber under microscopy with the PDB medium. Rice seeds were soaked in the conidial suspension for 48 h followed by drying in the air. The rice seeds were then treated with 11% FMA with a series of doses stated above. There were three replicates for each dose of a chemical. Each replicate contains five seedling trays. There were 100 g of rice seeds for every seedling tray.
Investigation of growth conditions of rice seedlings
Thirty days after sowing, 100 seedlings from every seedling tray were randomly selected to measure several growth indexes including the height of seedlings, number of roots (taproot and lateral root), the width of stem base and circularity of seedling (mass per centimeter of the seedling). Moreover, the dry weight of both underground and aboveground parts of the plant was measured after drying in an oven at 80 °C for 24 h in darkness.
Calculation of incidence and control efficiency of rice bakanae disease
To calculate the incidence of the rice bakanae disease during the seedling stage, seedlings withered to death or with an elongated stem and slender leaves were considered infected. The percentage of infected seedlings was equal to diseased plants divided by the whole rice seedlings. Continuously in the heading period, plants with poorly filled, melanized, or empty grains were counted as diseased plants. The ratio of the number of diseased plants to the number of areas investigated was considered as the percentage of infected plants. When calculating the control efficiency, the number of diseased seedlings or plants of the control treatment minus that of the chemical treatment. The difference value divided by the number of diseased plants was the control efficiency of the chemical. All experiments were conducted using a statistically randomized block design with three treatment replicates.
Yield measurement of rice coated by different doses of 11% FMA
Rice seedlings coated with every dose of 11% FMA were transplanted to the field with three replicates. At the maturation period, rice plants from every replicate of all treatments were gained. The paddy was then sun-dried and weighed. The values were converted to rice production per hectare for statistical analysis.
Confirmation of Fusarium fujikuroi
Rice seedlings suspected as infected were collected and brought back to the laboratory. Different parts of the seedlings, including leaves, stems, and roots were cut out with a sterilized scissor and put into a sterile petri dish followed by soaking in 3% NaClO for 5 min and in distilled water for 5 min twice successively. Then, samples were dried followed by being transferred to autoclaved PDA media and incubated at 28 °C for 24 h in darkness. Different diameter sizes of colonies grew on the medium and were transferred to new PDA plates.
When the diameter of the colonies was about 2 cm, the surface of the mycelia was flushed with 500 μL of distilled water to an agar water plate. After culturing for 2 h at 25 °C, the agar water plate was subject to single-cell isolation under the microscope. A single spore of F. fujikuroi was cultured at 25 °C for 5 days.
For the purified strain, DNA was extracted with the Takara kit and conducted PCR assay with Fusarium-specific primers ef1 (5’-ATGGGTAAGGAAGACAAGAC-3’) and ef2 (5’-GGAAGTACCAGTGATCATGTT-3’) and Fusarium fujikuroi-specific primer pair Fuji1F (5’-ACGTGTCAAACTAAACATTCGA-3’) and TEF1R (5’-GCGACAACATACCAATGACG-3’) [29,30]. The PCR products were performed by agarose gel electrophoresis to identify the fungus.
Data analysis
Data were subject to statistical analysis of variance using the SAS software. Significant differences between the two treatments were detected when the p - value was less than 0.05 (p < 0.05). Mean values with significant differences were labeled with different letters by T-test.
Hyphal growth was inhibited by 11% FMA
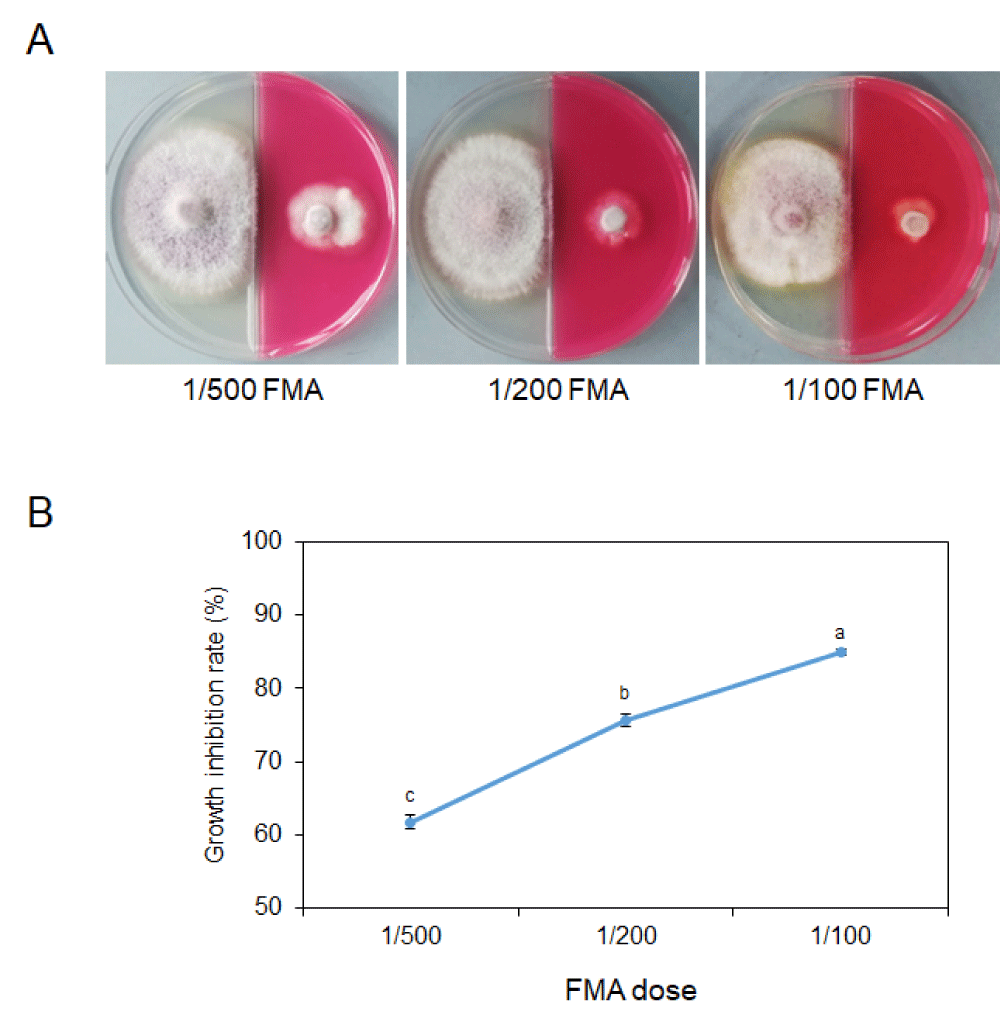
After culturing for 3 days, the diameter of the untreated colony was about 5 cm and the colony size of the 1/500 FMA treatment was 1.95 cm (Supplementary Table 1). As the dilution ratio decreases, the hyphal colony size becomes smaller (Figure 1A). When the dilution ratio was 1/200, the growth inhibition rate reaches 75.72% (Figure 1B, Supplementary Table 1). When 1/100 FMA is added into the PDA medium, the colony size decreases to only 0.76 cm, almost completely inhibited (Figure 1B, Supplementary Table 1).
These results demonstrated that FMA was effective in the prevention of the growth of the pathogen and this effect was a relation to the dosage of the chemical.
Appearance and safety test of seed coating using 11% FMA
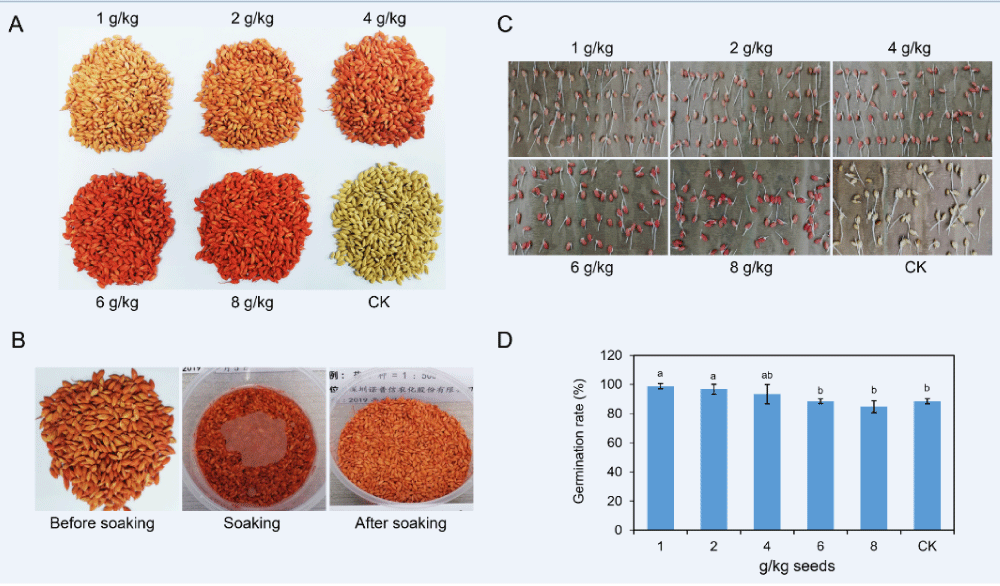
To conduct experiments indoors and in the field, we first applied a series of doses of 11% FMA to coat rice seeds. The coated seeds were pictured and shown in Figure 2A. Obviously, as the dose of chemical becomes higher, the red color of coated seeds exhibits darker. When 4 g of FMA was used to dress 1 kg of seeds, the color showed a relatively bright red. Moreover, the chemical with every dose performed with excellent uniformity onto the surface of the seeds (Figure 2A). As the rice seeds were associated with water in actual production, we then chose rice seeds dressed by the 2 g/kg chemical dose to examine resistance to soaking in water. Interestingly, no obvious discoloration was inspected among the coated seeds soaked in a bowl of water. This phenomenon was confirmed when the seeds were removed from soaking (Figure 2B).
Figure 1: Inhibition of 11% FMA on mycelial growth of Fusarium fujikuroi. (A) Colony of Fusarium fujikuroi in a binary petri dish after culturing for 3 days at 25 °C. Left, blank PDA medium; Right, PDA medium with diluted (1/500, 1/200 and 1/100) 11% FMA addition. (B) Line graph showing growth inhibition rates by 11% FMA with different dilution ratios. Significant differences are indicated by asterisks (T-test, p = 0.05).
Figure 2: Tentative identification of the secondary metabolites in six different isolates of S. sclerotiorum using UPLC-Q-TOF-ESI-MS.
When the coated seeds and untreated seeds were incubated on the kraft paper for 3 days, all seeds exhibited young roots and buds (Figure 2C). We calculated the germination rates of every treatment along with the control (uncoated seeds). At the dose of 1 g FMA for 1 kg of seeds, the germination rate was 98.8%. When the dose increased to 2 g FMA per 1 kg of seeds, we found the percentage was 96.7%. These were significantly greater than that of the CK, the germination rate of which was 88.5%. The germination rate decreased to 93.3%, 88.5%, and 84.7% as the pesticide dose increased to 4 g, 6 g and 8 g per 1 kg of rice seeds, respectively (Figure 2D). These data showed no significant difference compared with the germination rate of the CK. In conclusion, relatively low doses of FMA coating promoted germination while high doses might impede germination rates of rice seeds.
A certain dose of FMA coating improves the growth conditions of rice seedlings
Statistical analysis indicated that the underground parts of seedlings without seed coating, as pretreatment, had lower dry weights than those having their seeds treated with FMA. This result was consistent with the circularity index (Table 1). However, FMA treatments did not favor better performances than CK in other growth indexes including height, root number and width of stem base. Altogether, certain doses of FMA coating are safe for seedlings and improved underground growth.
| Table 1: Growth parameters of rice seedlings dressing with 11% FMA. | ||||||
| Doses (g/kg seeds) | Height {cm) | Number of roots | Dry weight of aboveground part {per 100 seedlings, g) | Dry weight of underground part {per 100 seedlings, g) | Width of stem base (mm) | Circularity of seedling (g/cm) |
| 1 | 14.1 a | 11.2 a | 2.41 a | 1.59 a | 2.11 a | 0.1712 ab |
| 2 | 14.4 a | 10.1 a | 2.21 a | 1.50 a | 2.28 a | 0.1541 b |
| 4 | 14.5 a | 9.5 a | 2.48 a | 1.44 ab | 2.40 a | 0.1786 a |
| 6 | 16.1 a | 10.1 a | 2.13 a | 1.49 a | 2.36 a | 0.1331 c |
| 8 | 15.5 a | 10.3 a | 2.48 a | 1.41 b | 2.35 a | 0.1562 b |
| CK(0) | 15.1 a | 11.0 a | 2.51 a | 1.36 c | 2.34 a | 0.1383 c |
FMA coating and rice bakanae disease control
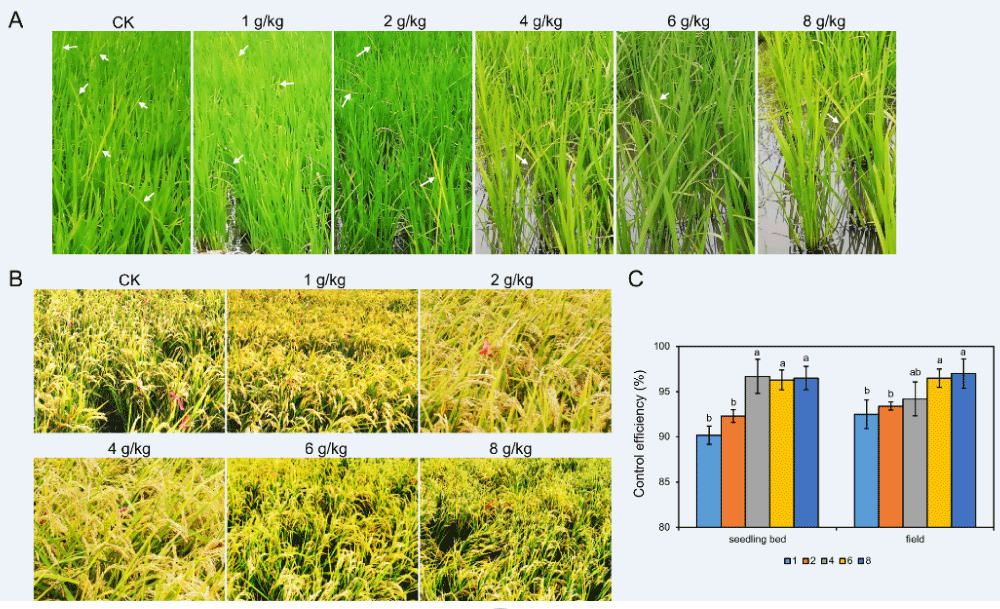
As the rice bakanae disease occurred both in the seedling bed and in the field, we evaluated disease control in relation to each FMA dose. While the CK exhibited more decayed or dead seedlings, seedlings treated by FMA showed a healthy stem base and few symptoms. As the dose of FMA increased, fewer diseased seedlings were found (Figure 3A). The disease control percentages of 4 g, 6 g, and 8 g FMA per 1 kg of rice seeds were over 95% in the seedling bed, significantly more than that of 1 g/kg or 2 g/kg (Figure 3C). This result indicated FMA coating had a special effect on seedling bakanae control. During the growing period, when in the field, rice plants with the FMA coating treatment showed better growth conditions than that of the CK, which had dramatically more infected plants by the rice bakanae pathogen (Figure 3B). Actually, an average of 75 diseased rice plants were found per 30 cm2 area in the CK, while there were less than 10 infected plants in all doses of FMA coating. The average numbers were 5.7, 5.0, 4.3, 2.3 and 2.3 for the dose of 1 g/kg, 2 g/kg, 4 g/kg, 6 g/kg, and 8 g/kg, respectively. The control effect of all the FMA treatments was more than 90%, especially in the 1:167 and 1:125 treatments, the percentages of both of which reached 96.9% (Supplementary Table 2 and Figure 3C). These results indicated that FMA coating is an effective approach to protect rice from bakanae disease during all growing periods.
Figure 3: Control efficiency of rice bakanae disease in the seedling and maturation stages. (A) Bakanae disease in rice seedlings. Arrows show infected seedlings. (B) Rice bakanae disease at the maturation stage. Infected plants were tied with red ropes. (C) Histogram showing control efficiency of different doses of FMA dressing in the seedling and maturation stage.
FMA coating can improve rice yield
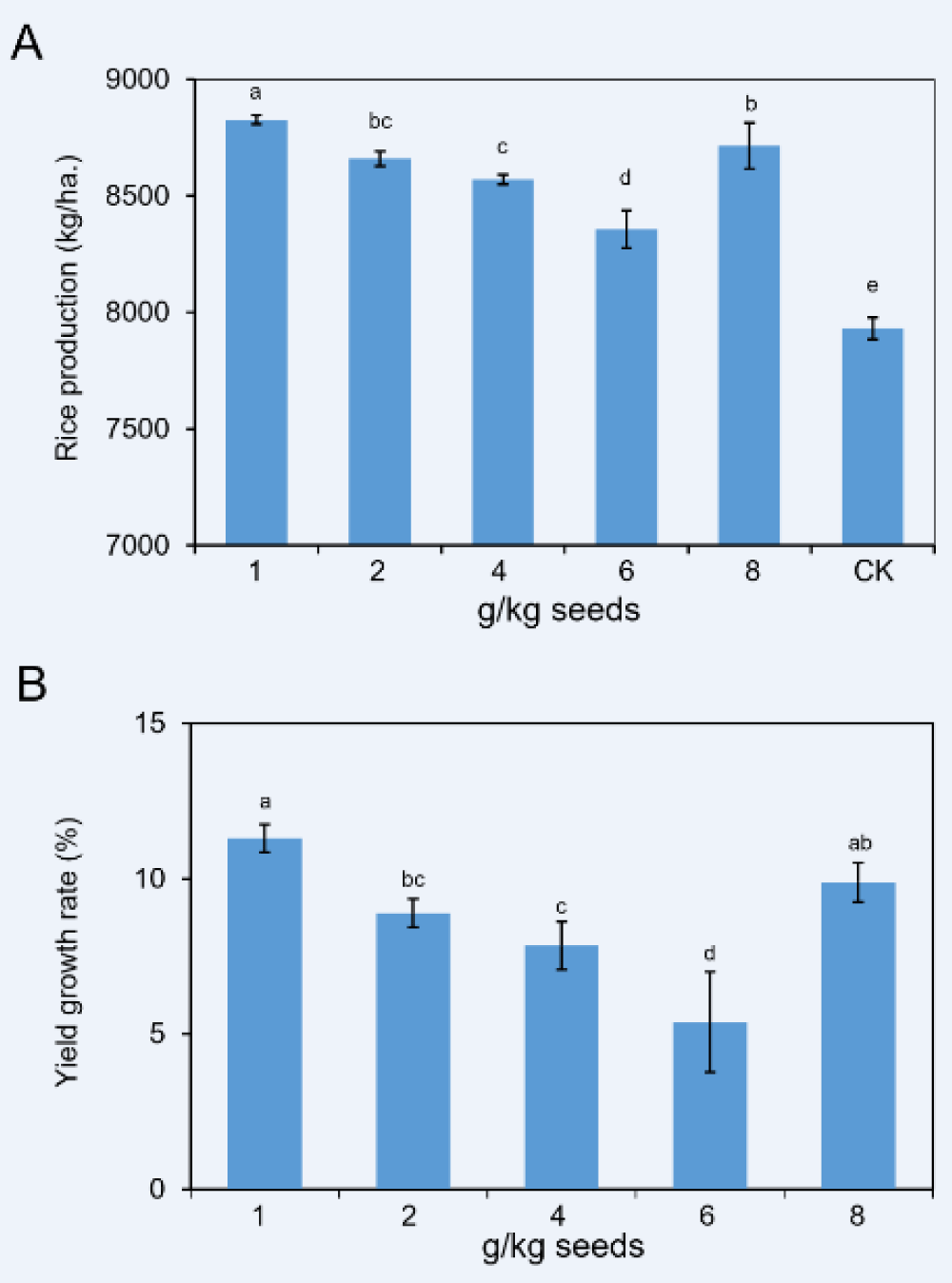
At the maturation stage, the yield for every treatment was measured. Rice seeds coated with 11% FMA at 1 g/kg dose could produce an average of 8826.7 kg of rice per hectare, the highest yield among all treatments and significantly more than those of other treatments. The yield of 2 g/kg, 4 g/kg, 6 g/kg, and 8 g/kg treatments were 8659.4, 8570.6, 8357.5 and 8714.8 kg/ha, respectively (Supplementary Table 3). Statistical analysis showed there was no significant difference between that 8 g/kg and 2 g/kg. However, both products of the two doses exhibited extremely higher than that of 6 g/kg. Interestingly, the yield of the CK treatment was only 7930.8 kg/ha, dramatically less than those of all FMA coating treatments (Figure 4A).
We then calculated the yield increase rates for every treatment. Except for the 6 g/kg treatment, the increase rates of others were all around 10% (Supplementary Table 3), which was a notable value in the field. Amongst, the percentage of the 1 g/kg dose was significantly more notable than those of other doses. Besides the 8 g/kg treatment, as the coating dose decreased, the yield increase rates showed more obvious (Figure 4B). Based on these values, we speculated that FMA coating contributed to improving rice yield at a certain range of doses.
Figure 4: Rice production and yield growth rates of plants treated by 11% FMA. (A) Comparison of rice production with doses of FMA dressing. (B) Statistic analysis of growth rates of rice production in comparison with the CK.
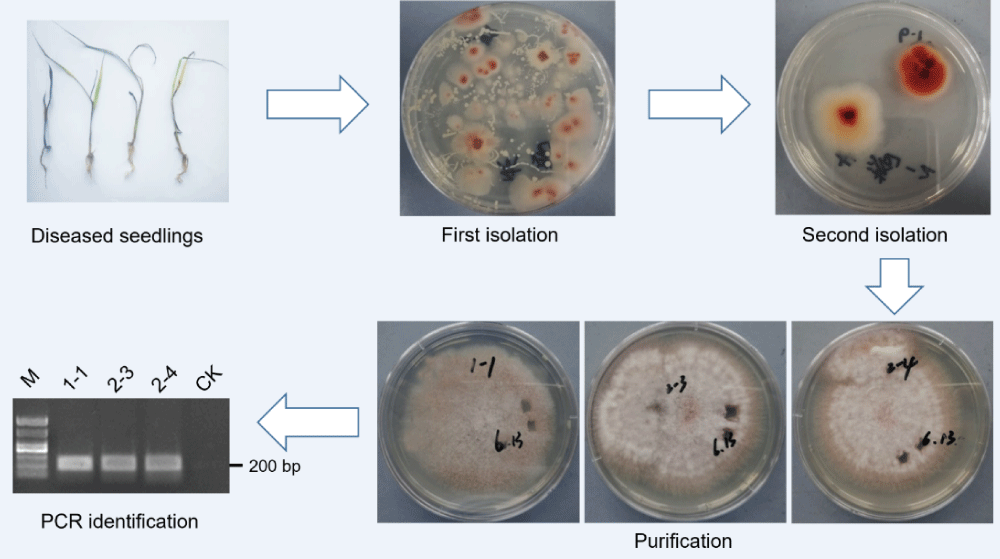
Identification of rice bakanae disease from the seedling bed
Several infected rice seedlings were picked to isolate pathogens (Figure 5A). Plenty of colonies considered as a genus were isolated on the PDA medium (Figure 5B). These colonies were isolated to a species and purified into three replicates (Figures 5C and 5D). The mycelial DNA of all replicates was extracted and identified. We used the Fusarium-specific primer for PCR for the first screening. Both the samples showed positive bands, which means they belong to Fusarium. We then used the Fusarium fujikuroi-specific primer to second identification. All the DNA samples of the three candidate colonies showed a 200 bp band in electrophoresis (Figure 5E), which indicated that the pathogen detected was F. fujikuroi. These results confirmed that the disease occurrence in the field test was rice bakanae disease.
Figure 5: Isolation, purification and identification of Fusarium fujikuroi from the field. (A) Diseased plants from the seedling bed. (B) Fungus isolated and colony grew after culturing for 24 h. (C) Isolated colony growth after culturing for 36 h. (D) Candidate colony growth of 4 days after single-cell purification. (E) PCR and electrophoresis identification of Fusarium fujikuroi. M-marker; CK-distilled water as the template.
Different from other common rice diseases, including rice blast, rice false smut and rice sheath blight, rice bakanae is a typical seed-borne disease [4,31-33]. In practice, most methods used to control rice bakanae focu on underground parts. However, there was no significant difference in germination rates between seeds treated by soaking in 500 g/L fluazinam SC and untreated (control) seeds [16]. When an endophytic bacterium, Bacillus oryzicola YC7007, the suspension was used to drench the root to prevent rice bakanae, the strain was capable of inducing systemic resistance against the pathogen [25]. Among the methods used to control the disease, seed dressing or soaking has become more popular. Fungicides commonly used for seed treatment were carbendazim, prochloraz and thiram [7,14,15]. For a while, treating seeds with these fungicides exhibited impressive results. However, due to the abuse of these chemicals, resistant isolates of F. fujikuroi have evolved. In the last few years, the availability of authorized chemicals for seed dressing decreased but the need to control rice bakanae disease was still considerable. Seeds free from F. fujikuroi are produced or stored by seed companies, but developing novel fungicides for seed dressing has become necessary [7].
In this study, three compounds including fludioxonil, metalaxyl-M, and azoxystrobin combined at the ratio of 1:3:6, into a mixture named11% FMA. In our company, this chemical had been a well-selling pesticide product that is registered on several crops including peanuts, potatoes and corn by seed coating. The according to target diseases were peanut root rot, potato black scurf, and corn stalk rot. We are now trying to expand the registration to rice bakanae disease through kinds of experiments and reports. The pesticide registration number is PD20150418 and the expiry date is March 2025.
The safety of seeds has been the most important and fundamental requirement for a seed-coating formulation. In our study, rice seeds coated with 11% FMA atdoses ranging from 1 g/kg to 4 g/kg showed extremely high germination rates, which exceed 93%. Despite the germinate rate decreasing at the dose of 6 g/kg and 8 g/kg, the percentages approach that of the untreated seeds. Appearance after the coating is an index concerning the quality of a seed-coating chemical. In this study, 11% FMA exhibited high uniformity on the surface of rice seeds after dressing. This characteristic insures sufficient function of the chemical during the growing period of crops. Moreover, treated rice seeds gain strong resistance to water soaking and show less discoloration. Thus, 11% FMA can apply to kinds of rice areas with different cultivation patterns around the world.
An effective period of 11% FMA depends on the action modes of the three compounds. As fludioxonil is a broad-spectrum fungicide with a higher effect and lower toxicity. It has control efficiency on lots of pathogens including basidiomycetes, ascomycetes and Deuteromycetes. Moreover, fludioxonil can restrain the transmission of pathogens through the seeds and soil. Metalaxyl-M specifically aims at controlling oomycetes and thus, can protect rice seeds or seedlings from lower fungal pathogens at the early stage. Furthermore, metalaxyl-M can conduct efficiently from the seed and root to the aboveground part of the plant. With a wide prevention spectrum and high permeability, the systemic chemical azoxystrobin act by disturbing mitochondrial and respiration in many fungi, including Fusarium. Altogether, a combination of these three chemicals could durably prevent the rice bakanae.
The field trial is an expanding test of the indoor experiment of chemical effect determination. It is an important and necessary step since it simulates production in practice. In this study, the whole growing period was tracked to investigate the impact on rice bakanae control and final production by 11% FMA coating. The seedling stage is one of the growth phases when the plant is more susceptible to rice bakanae disease. Our study demonstrated that the control efficiency of every dose of 11% FMA dressing was greater than 90%, suggesting sporadic bakanae disease occurrence in the seedling bed. This result coincided with the effect of seed dressing with fungicides, which is best in the early stage. Another situation was that rice plants survived in the seedling period and exhibited symptoms after the heading period. During the maturation period, with dozens of diseased plants appearing in the control treatment, we calculated the control efficiency from 92.5% at 1 g/kg to more than 95% at 6 g/kg or at 8 g/kg. To some extent, the control effect increased as the dose of FMA application increased but the differences were quite slight. However, rice yield is the most important agricultural trait. The circularity of seedings was basically consistent with the rice yield. The growth rate extremely increased at 1 g/kg, 8 g/kg and 2 g/kg treatment. Considering the cost of chemical application in the field, the optimal dose range of 11% FMA was recommended as 1 g/kg to 2 g/kg.
As with the effect on rice production, our study showed that seeds dressed with different doses of 11% FMA could produce more than 8000 kg of rice per hectare. While the production of CK was 7930.8 kg/ha. These figures demonstrate that production loss is associated with the occurrence of bakanae disease and therefore, it is of great economic value to use 11% FMA to dress rice seeds. In this way, a novel solution to rice bakanae disease was developed to guarantee the production of rice worldwide.
This work was supported by Science and Technology Plan Project of Guangdong Province (2021B1212050011), Science and Technology Plan Project of Shenzhen (JSGG20170822153048662), and Science and Technology Plan Project of Shenzhen (JSGG20210802154804014).
Deng Chen conducted most experiments and wrote the article. Qi-Juan Hu participated in the bioassay of this solution. Xiao-Feng Chen was totally in charge of the project and the article revision. We thank Professor Juan-Wei Mu and Zhi-An Lun, from the Heilongjiang Academy of Land Reclamation Sciences, for their experimental investigation in the field.
- Hsieh WH, Smith SN, Snyder WC. Mating groups in Fusarium moniliforme. Phytopathol. 1977; 67:1041-1043.
- Ou SH. Rice Diseases. Second edition. Slough, UK: CAB International. 1987.
- Singh R, Sunder S. Footrot and bakanae of rice: retrospects and prospects. Int. J. Trop. Plant Disease. 1997; 15:153-176.
- Mew TW, Gonzales P. Seed-borne fungi causing stem, leaf sheath, and root diseases in rice. In: A handbook of rice seed-borne fungi, eds. by TW Mew and P Gonzales. 2002; 31–34. Science Publishers, Enfield, NH, USA. International Rice Research Institute, Makati, Philippines.
- Wulff EG, Sørensen JL, Lübeck M, Nielsen KF, Thrane U, Torp J. Fusarium spp. associated with rice Bakanae: ecology, genetic diversity, pathogenicity and toxigenicity. Environ Microbiol. 2010 Mar;12(3):649-57. doi: 10.1111/j.1462-2920.2009.02105.x. Epub 2009 Nov 25. PMID: 20002135.
- Hsuan HM, Salleh B, Zakaria L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int J Mol Sci. 2011;12(10):6722-32. doi: 10.3390/ijms12106722. Epub 2011 Oct 11. PMID: 22072914; PMCID: PMC3211005.
- Matic S, Gullino ML, Spadaro D. The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. Front Biosci (Elite Ed). 2017 Jun 1;9(2):333-344. doi: 10.2741/e806. PMID: 28410155.
- Ito S, Kimura J. Studies on the bakanae disease of the rice plant. Rep. Hokkaido Natl. Agric. Exp. Stat. 1931; 27:1-95.
- Amoah BK, Rezanoor HN, Nicholson P, MacDonald MV. Variation in the Fusarium section Liseola: pathogenicity and genetic studies of Fusarium moniliforme Sheldon from different hosts in Ghana. Plant Pathol. 1995; 44:563-572.
- Ogawa K. Damage by bakanae disease and its chemical control. Jpn. Pestic. Info. 1988; 52:13-15.
- Cao DD, Ruan XL, Zhan Y, Xu JF. Control Efficiency of Different Treatments on Rice Bakanae Disease. Plant Diseases and Pests. 2015; 6:31-34.
- Chen Z, Gao T, Liang S, Liu K, Zhou M, Chen C. Molecular mechanism of resistance of Fusarium fujikuroi to benzimidazole fungicides. FEMS Microbiol Lett. 2014 Aug;357(1):77-84. doi: 10.1111/1574-6968.12504. Epub 2014 Jul 7. PMID: 24913566.
- Li M, Li T, Duan Y, Yang Y, Wu J, Zhao D, Xiao X, Pan X, Chen W, Wang J, Chen C, Zhou M. Evaluation of Phenamacril and Ipconazole for Control of Rice Bakanae Disease Caused by Fusarium fujikuroi. Plant Dis. 2018 Jul;102(7):1234-1239. doi: 10.1094/PDIS-10-17-1521-RE. Epub 2018 Apr 25. PMID: 30673573.
- Park WS, Choi HW, Han SS, Shin D, Shim HK, Jung ES, Lee SW, Lim CK, Lee YH. Control of bakanae disease of rice by seed soaking into the mixed solution of prochloraz and fludioxonil. Res. Plant Dis. 2009; 15:94-100.
- Kim SH, Park MR, Kim YC, Lee SW, Choi BR, Lee SW, Kim IS. Degradation of prochloraz by rice bakanae disease pathogen Fusarium fujikuroi with differing sensitivity: a possible explanation for resistance mechanism. J. Korean Soc. Appl. Biol. Chem. 2010; 53:433-439.
- Qu XP, Li JS, Wang JX, Wu LY, Wang YF, Chen CJ, Zhou MG, Hou YP. Effects of the dinitroaniline fungicide fluazinam on Fusarium fujikuroi and rice. Pestic Biochem Physiol. 2018 Nov;152:98-105. doi: 10.1016/j.pestbp.2018.09.010. Epub 2018 Sep 20. PMID: 30497718.
- Tateishi H, Saishoji T, Suzuki T, Chida T. Antifungal properties of the seed disinfectant miconazole and its protection against “bakanae” and other diseases of rice. Jpn. J. Phytopathol. 1998; 64:443-450.
- Hou YP, Qu XP, Mao XW, Kuang J, Duan YB, Song XS, Wang JX, Chen CJ, Zhou MG. Resistance mechanism of Fusarium fujikuroi to phenamacril in the field. Pest Manag Sci. 2018 Mar;74(3):607-616. doi: 10.1002/ps.4742. Epub 2017 Nov 27. PMID: 28960890.
- FRAC. Fungicide Resistance Action Committee. Crop Life Intl. Brussels, Belgium. https://www.frac.info. 2020.
- He Y, Jiao X, Zhang T, Wang M, Khan M, Khan MR, She Y. Study on the dissipation pattern and risk assessment of metalaxyl-M in rice grains and paddy soil and water by liquid chromatography-tandem mass spectrometry. Environ Sci Pollut Res Int. 2021 Jan;28(4):4245-4252. doi: 10.1007/s11356-020-10802-3. Epub 2020 Sep 16. PMID: 32939654.
- Jimenez JJ, Bernal JL, del Nozal MJ, Bernal J, Toribio L. Persistence and degradation of metalaxyl, lindane, fenvalerate and deltamethrin during the wine making process. Food Chem. 2007; 104:216-223.
- Karras G, Savvas D, Patakioutas G, Pomonis T, Albanis T, Pomonis P. Modelling the transport of metalaxyl in gerbera plants grown in a closed-loop hydroponic system. Biosyst. Eng. 2007; 96:279-292.
- Monkiedje A, Spiteller M, Maniepi SJN, Sukul P. Influence of metalaxyl- and mefenoxam-based fungicides on chemical and biochemical attributes of soil quality under field conditions in a southern humid forest zone of Cameroon. Soil Biol. Biochem. 2007; 39:836-842.
- Liang S., Xu X., Lu Z., 2018. Effect of azoxystrobin fungicide on the physiological and biochemical indices and ginsenoside contents of ginseng leaves. J. Ginseng Res. 42, 175-182.
- Hossain MT, Khan A, Chung EJ, Rashid MH, Chung YR. Biological Control of Rice Bakanae by an Endophytic Bacillus oryzicola YC7007. Plant Pathol J. 2016 Jun;32(3):228-41. doi: 10.5423/PPJ.OA.10.2015.0218. Epub 2016 Jun 1. PMID: 27298598; PMCID: PMC4892819.
- Sauter H, Steglich W, Anke T. Strobilurins: Evolution of a New Class of Active Substances. Angew Chem Int Ed Engl. 1999 May 17;38(10):1328-1349. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1328::AID-ANIE1328>3.0.CO;2-1. PMID: 29711574.
- Gisi U. Synergistic interaction of fungicides in mixtures. Phytopathol. 1996; 86:1273-1279.
- Abdel-Ghani AH, Sanchez DL, Kumar B, Lubberstedt T. Paper Roll Culture and Assessment of Maize Root Parameters. Bio-protoc. 2016; 6:e1926. DOI: 10.21769/BioProtoc.1926.
- Amatulli MT, Spadaro D, Gullino ML, Garibaldi A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010; 59:839-844.
- Amatulli MT, Spadaro D, Gullino ML, Garibaldi A. Conventional and real-time PCR for the identification of Fusarium fujikuroi and Fusarium proliferatum from diseased rice tissues and seeds. Eur. J. Plant Pathol. 2012; 134:401-408.