More Information
Submitted: December 04, 2023 | Approved: December 11, 2023 | Published: December 12, 2023
How to cite this article: Tsygankova VA, Andrusevich Ya V, Vasylenko NM, Pilyo SG, Klyuchko SV, et al. Screening of Auxin-like Substances among Synthetic Compounds, Derivatives of Pyridine and Pyrimidine. J Plant Sci Phytopathol. 2023; 7: 151-156.
DOI: 10.29328/journal.jpsp.1001121
Copyright License: © 2023 Tsygankova VA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Phaseolus vulgaris L.; Auxins; IAA; NAA; Pyridine; Pyrimidine; Ivin; Methyur; Kamethur; Rooting of plant cuttings
Screening of Auxin-like Substances among Synthetic Compounds, Derivatives of Pyridine and Pyrimidine
Tsygankova VA* , Andrusevich YaV, Vasylenko NM, Pilyo SG, Klyuchko SV and Brovarets VS
, Andrusevich YaV, Vasylenko NM, Pilyo SG, Klyuchko SV and Brovarets VS
Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, National Academy of Sciences of Ukraine, 1, Academician Kukhar str., 02094, Kyiv-94, Ukraine
*Address for Correspondence: Dr. Tsygankova VA, Biol. Sci., Principal researcher, Senior Staff Scientist, Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, National Academy of Sciences of Ukraine, 1, Academician Kukhar str., 02094, Kyiv-94, Ukraine, Email: vTsygankova@ukr.net
The effect of known synthetic compounds Ivin (N-oxide-2,6-dimethylpyridine), Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine), Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine) and new synthetic compounds, derivatives of pyrimidine (No. 1 - 7) on the rooting of isolated stem cuttings of haricot bean (Phaseolus vulgaris L.) variety Bilozernaya was studied. The growth regulatory activity of synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1 - 7) was compared with the activity of auxins IAA (1H-indol-3-yl)acetic acid) and NAA (1-naphthylacetic acid). The conducted studies showed that the regulatory effect of synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyridine (No. 1 - 7) on the rooting of isolated stem cuttings of haricot bean was similar to the auxins IAA and NAA. The synthetic compounds Ivin, Methyur, and Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1, 4, 5, and 7) showed the highest auxin-like activity. The indicators of the total number of roots (pcs) and total length of roots (cm) obtained on isolated stem cuttings of haricot bean immersed in a water solution of synthetic compounds Ivin, Methyur, Kamethur and synthetic compounds, derivatives of pyridine (No. 1, 4, 5 and 7), used at a concentration of 10-7 M, statistically significantly exceeded similar indicators obtained on control isolated stem cuttings of haricot bean immersed in distilled water. The practical use of synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1, 4, 5 and 7) is proposed to improve the vegetative propagation of haricot bean plants (Phaseolus vulgaris L.) and other plant species of the family Fabaceae by stem cuttings.
Auxins and cytokinins are known as the main phytohormones that play a key role in the formation of plant organs during embryogenesis and their subsequent growth and development at the post-embryonic stage [1-4].
Auxins, together with cytokinins, control the formation of primary shoots and root apical meristems during plant embryogenesis, and the subsequent formation of the main roots, leaves, and flowers from lateral or secondary meristems at the postembryonic stage [1-5]. Today, natural auxins such as IAA (indole-3-acetic acid), 4-Cl-IAA (4-chloro-IAA), PAA (phenylacetic acid), IPA (indole-3-pyruvic acid), IBA (indole-3-butyric acid) or synthetic auxins such as NAA (1-naphthylacetic acid), 2,4-D (2,4-dichlorophenoxyacetic acid), 3,4-D (3,4-dichlorophenoxyacetic acid), 2,4,5-T (2,4,5-trichlorophenoxyacetic acid), 4-CPA (4-chloropheno-xyacetic acid), dicamba (3,6-dichloro-2-methoxybenzoic acid), picloram (4-amino-3,5,6-trichloro-pyridine-2-carboxylicacid), BSAA (3-(benzo[b]selenienyl)acetic acid), 5,6-Cl2-IAA-Me (5,6-dichloroindole-3-acetic acid methyl ester), TA-12 (1-[2-chloroethoxycarbonyl-methyl]-4-naphthalenesulfonic acid calcium salt), TA-14 (1-[2-dimethyl-aminoethoxicarbonylmethyl] naphtalene chlormethylate), as well as natural cytokinin such as zeatin or synthetic cytokinins such as kinetin (6-furfurylaminopurine), 2iP (N6-(2-isopentenyl)adenine), BA (N6-benzyladenine), BAP (6-benzylaminopurine), BPA (N-benzyl-9-(2-tetrahydro-pyranyl)-adenine), TDS (thidiazuron) are widely used as plant growth regulators [6-17].
Nevertheless, a very urgent task for plant biotechnology, agriculture, horticulture, and landscape architecture is the search for new synthetic compounds that exhibit activity similar to auxins and cytokinins to improve the vegetative propagation of plants by stem cuttings and microtonal propagation of plants in vitro [18-22].
Recently, new classes of synthetic compounds based on low molecular weight heterocyclic compounds, derivatives of pyridine, pyrimidine, pyrazole, triazine, oxazole, oxazolopyrimidine, and isoflavonoids have also been tested for growth regulatory effects similar to auxins and cytokinins [23-27]. The most promising among these classes of synthetic compounds for practical use are pyridine and pyrimidine derivatives, which are used in agriculture as herbicides, fungicides, and plant growth-regulating agents [28-32]. The main advantage of using pyridine and pyrimidine derivatives is the broad specificity of their regulatory effects on the growth and development of different plant species and varieties during ontogenesis and on the organogenesis of plant shoots and roots in vitro in low concentrations from 10-5 M to 10-9 M, which do not have toxic effects for humans, animals and the environment [33-37].
The main goal of this work is the screening of synthetic compounds, derivatives of pyridine and pyrimidine as new effective substitutes for phytohormones auxins to improve the vegetative propagation of haricot bean plants (Phaseolus vulgaris L.) by stem cuttings.
A study of auxin-like activity of synthetic compounds, derivatives of pyrimidine was carried out using a specific bioassay for rooting of isolated (separated from the roots) stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya [27,38]. It is known that this bioassay is based on the key role of auxins in the regulation of the formation and growth of adventitious roots on stem cuttings and leaf petioles of plants.
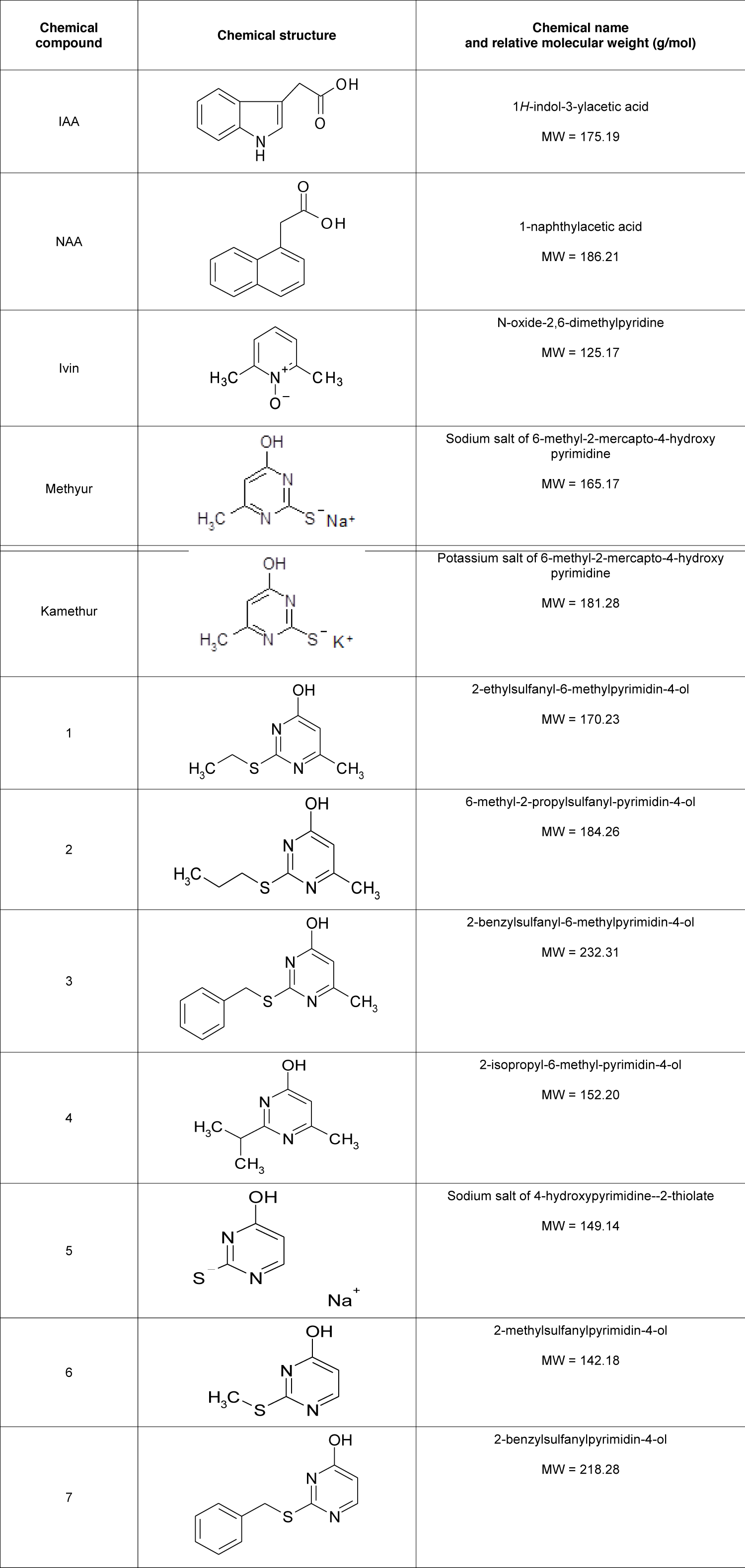
Synthetic compounds Ivin (N-oxide-2,6-dimethylpyridine), Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxy-pyrimidine), Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine) and synthetic compounds, derivatives of pyrimidine (No. 1 - 7) were synthesized at the Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine (Table 1).
Table 1: The effect of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1 - 7), used at a concentration of 10-7M, on the rooting of isolated stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya for 4 weeks compared to control isolated stem cuttings of haricot bean (K).
The growth regulatory activity of synthetic compounds, derivatives of pyridine and pyrimidine was compared with the activity of auxins IAA (1H-indol-3-yl)acetic acid) and NAA (1-naphthylacetic acid) manufactured by Sigma-Aldrich, USA (Table 1).
The seeds of haricot bean (P. vulgaris L.) variety Bilozernaya were sterilized with 1% KMnO4 solution for 5 - 10 min, then treated with 96% ethanol solution for 1 min, after which they were washed three times with sterile distilled water. After this procedure, seeds were placed in the plastic cuvettes (each containing 15 - 20 seeds) on the perlite moistened with distilled water. Then seeds were placed in the thermostat for their germination in darkness at the temperature of 23 °C for 48 h. After the emergence of sprouts, they were placed in a climatic chamber in which seedlings were grown for 10 days at 16/8 h light/dark conditions, at the temperature of 23 °C - 25 °C, light intensity of 3000 lux, and air humidity of 60% - 80%. To stimulate the formation of adventitious roots on stem cuttings isolated from haricot bean seedlings, they were cut at a distance of 5 mm - 10 mm from the roots and then were placed immediately to a depth of 30 cm into separate glass conical flasks with a volume of 100 ml containing either distilled water (control samples) or water solution of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur and synthetic compounds, derivatives of pyrimidine (No. 1 - 7), used in a concentration of 10-7 М (experimental samples). After 4 weeks, the indicators of the average total number of roots (pcs) and the total length of roots (cm) obtained on experimental isolated stem cuttings of haricot bean were determined and compared with similar indicators obtained on control isolated stem cuttings of haricot bean.
Statistical processing of the experimental data, performed in triplicate, was carried out according to the Student’s t-test with a significance level of p ≤ 0.05; mean values ± standard deviation (± SD) [39].
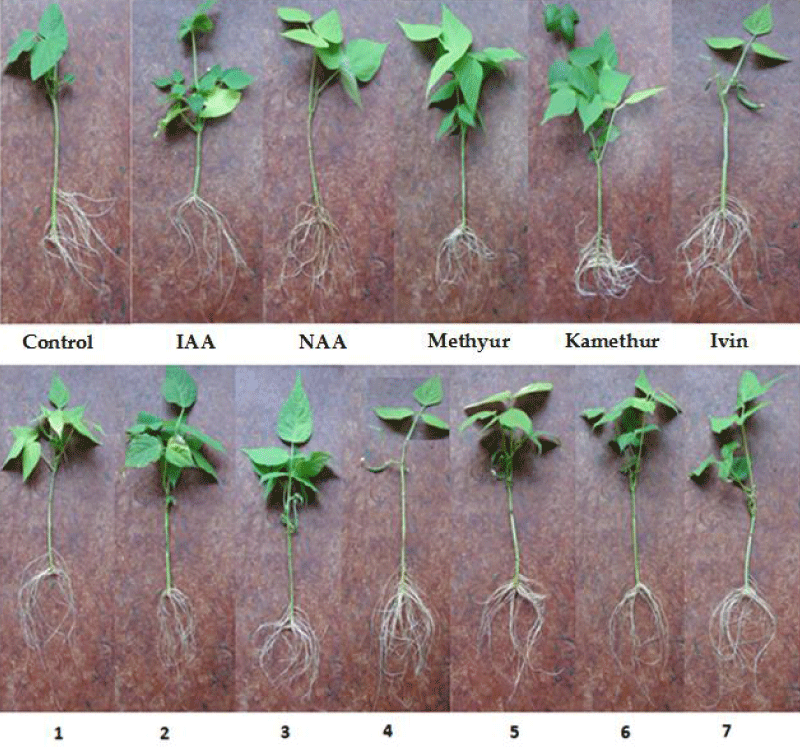
The conducted studies showed that synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1 - 7) revealed a regulatory effect on the rooting of isolated stem cuttings of haricot bean, similar to the auxins IAA and NAA (Figure 1).
Figure 1: The effect of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1 - 7), used at a concentration of 10-7M, on the rooting of isolated stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya for 4 weeks compared to control isolated stem cuttings of haricot bean (K).
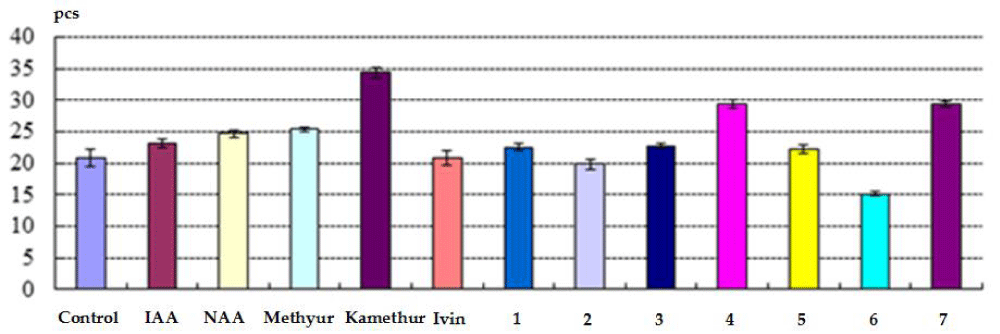
The indicators of the total number of roots (pcs) obtained on isolated stem cuttings of haricot bean immersed in a water solution of auxins IAA and NAA, synthetic compounds Methyur, Kamethur and synthetic compounds, derivatives of pyrimidine (No. 1, 3, 4, 5 and 7), which were used at a concentration of 10-7 M, exceeded similar indicators obtained on isolated stem cuttings of haricot bean immersed in distilled water (control) (Figure 2).
Figure 2: The effect of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur and synthetic compounds, derivatives of pyrimidine (No. 1 - 7), used at a concentration of 10-7 M, on indicators of the total number of roots (pcs) obtained on isolated stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya for 4 weeks compared to control isolated stem cuttings of haricot bean (K).
Auxins IAA and NAA, as well as synthetic compounds Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 4 and 7) revealed the highest regulatory activity. The indicators of the total number of roots (pcs) obtained on isolated stem cuttings of haricot beans statistically significantly increased: by 11% - after treatment with IAA, by 19% - after treatment with NAA, by 22% - after treatment with Methyur, by 65% - after treatment with Kamethur, and by 41% - 42% - after treatment with synthetic compounds, pyrimidine derivatives (No. 4 and 7), compared to similar indicators obtained on control isolated stem cuttings of haricot bean immersed in distilled water (Figure 2).
It was also found that the activity of synthetic compounds Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 4 and 7) was higher than the activity of auxins IAA and NAA. The indicators of the total number of roots (pcs) obtained on isolated stem cuttings of haricot bean after treatment with synthetic compounds Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 4 and 7) statistically significantly exceeded: by 11% - 54% the similar indicators obtained on isolated stem cuttings of haricot bean after treatment with auxin IAA and by 3% - 46% the similar indicators obtained on isolated stem cuttings of haricot bean after treatment with auxin NAA, respectively (Figure 2).
Synthetic compounds, derivatives of pyrimidine (No. 1, 3, 5) showed less activity, indicators of the total number of roots (pcs) obtained on isolated stem cuttings of haricot bean after treatment with these compounds statistically significantly increased by 7% - 10%, compared to similar indicators obtained on control isolated stem cuttings of haricot bean immersed in distilled water (Figure 2).
At the same time, indicators of the total number of roots (pcs) obtained on isolated stem cuttings of haricot beans immersed in a water solution of the synthetic compound Ivin and synthetic compounds, derivatives of pyrimidine (No. 2 and 6), which were used at a concentration of 10-7 M, almost did not statistically significantly exceed or were statistically significantly lower than similar indicators obtained on control isolated stem cuttings of haricot bean immersed in distilled water (Figure 2).
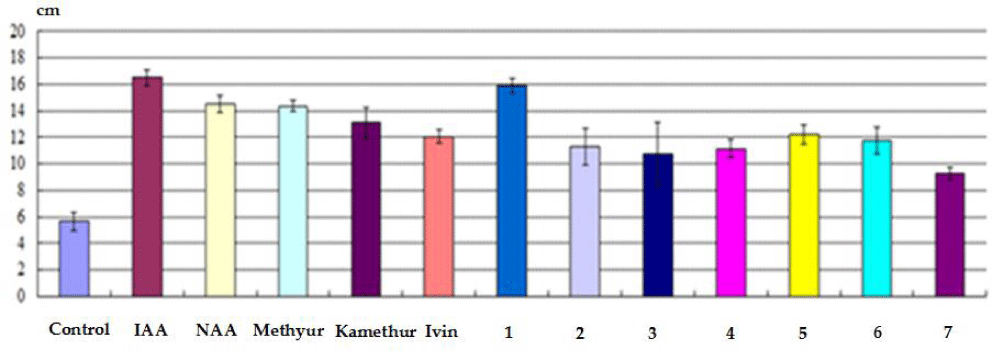
The conducted studies also showed that the indicators of the total length of roots (cm) obtained on isolated stem cuttings of haricot beans immersed in a water solution of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur and synthetic compounds, derivatives of pyrimidine (No. 1 - 7), which were used at a concentration of 10-7 M, exceeded similar indicators obtained on isolated stem cuttings of haricot bean immersed in distilled water (control) (Figure 3).
Figure 3: The effect of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur and synthetic compounds, derivatives of pyrimidine (No. 1 - 7), used at a concentration of 10-7 M, on indicators of the total length of roots (cm) obtained on isolated stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya for 4 weeks compared to control isolated stem cuttings of haricot bean (K).
The highest regulatory activity was shown by the auxins IAA and NAA, as well as synthetic compounds Ivin, Methyur, and Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1, 5, and 6). The indicators of the total length of roots (cm) obtained on isolated stem cuttings of haricot beans statistically significantly increased: by 191% - after treatment with IAA, by 156% - after treatment with NAA, by 153% - after treatment with Methyur, by 131% - after treatment with Kamethur, by 113% - after treatment with Ivin, and by 108 - 181% - after treatment with synthetic compounds, pyrimidine derivatives (No. 1, 5 and 6), compared to similar indicators obtained on control isolated stem cuttings of haricot bean immersed in distilled water (Figure 3).
Synthetic compounds, derivatives of pyrimidine (No. 2, 3, 4 and 7) showed less activity, indicators of the total length of roots (cm) obtained on isolated stem cuttings of haricot bean after treatment with these compounds statistically significantly increased by 63% - 100%, compared to similar indicators obtained on control isolated stem cuttings of haricot bean immersed in distilled water (Figure 3).
It was also found that the activity of the synthetic compound, a derivative of pyrimidine (No. 1) exceeded the activity of auxin NAA. The indicators of the total length of roots (cm) obtained on isolated stem cuttings of haricot beans after treatment with this compound statistically significantly exceeded by 25 % similar indicators obtained on isolated stem cuttings of haricot beans after treatment with auxin NAA (Figure 3).
Thus, the obtained data indicate that synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1, 4, 5 and 7) revealed high auxin-like activity on the rooting of isolated stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya. Synthetic compounds, and derivatives of pyrimidine (No. 2, 3, and 6) showed less activity.
Summarizing the results obtained, it should be noted that synthetic compounds Ivin, Methyur, and Kamethur were studied in our previous works [35-37], which showed their both auxin-like and cytokinin-like regulatory effects on the growth and development of various crops during ontogenesis and organogenesis of plant shoots and roots in vitro in concentrations from 10-5 M to 10-7 M.
In work [35], we investigated the use of synthetic compounds Ivin, Methyur, and Kamethur to increase the yield of grain sorghum (Sorghum bicolor L.) and sweet sorghum (Sorghum saccharatum L.) of different varieties. It was shown that the yield indicators (panicle length and fresh grain weight) of sorghum plants grown for 4 months in the field, treated with Ivin, Methyur, and Kamethur at a concentration of 10-7 M, exceeded the yield indicators of control plants treated with distilled water. Based on the obtained results, a conclusion was made about the high growth regulatory effect of synthetic compounds Ivin, Methyur, and Kamethur, similar to phytohormones auxins and cytokinins.
In work [36], we studied the effect of synthetic compounds Ivin, Methyur, and Kamethur on the growth and productivity of the sunflower (Helianthus annuus L.) variety Bastion. It was shown that the growth regulatory activity of synthetic compounds Ivin, Methyur, and Kamethur was similar to or higher than the growth regulatory activity of auxin IAA. It was found that the treatment of seeds before planting in the soil with synthetic compounds Ivin, Methyur and Kamethur at a concentration of 10-7 M contributes to an increase in morphological parameters (length of shoot and root, fresh weight of plant and basket) and biochemical parameters (content of chlorophylls a and b, and carotenoids) of sunflower (H. annuus L.) variety Bastion, grown in field conditions for 3 months.
In work [37], we studied the effect of synthetic compounds Ivin, Methyur, and Kamethur on the organogenesis of shoots and roots of the miniature rose (Rosa mini L.) in vitro. It is shown that the effect of synthetic compounds Ivin, Methyur, and Kamethur, which were used in concentrations of 10-5 M, 10-6 M, and 10-7 M per 1 liter of MS medium (Murashige and Skoog) on the organogenesis of shoots and roots of miniature rose (R. mini L.) in vitro is similar or higher than the effect of the plant hormone auxin IAA used in the same concentrations. Synthetic compounds Ivin, Methyur, and Kamethur showed the greatest influence on the organogenesis of shoots and roots of the miniature rose (R. mini L.) in vitro when applied in concentrations: Ivin in concentrations of 10-5 M and 10-6 M, Kamethur in concentrations of 10-5 M and 10-6 M, Methyur in concentrations of 10-5 M and 10-7 M. The plant hormone auxin IAA showed the greatest effect on the organogenesis of shoots and roots of miniature rose (R. mini L.) in vitro when used as a component of MS medium in concentrations of 10-6 M and 10-7 M per 1 liter of MS medium. The obtained results confirmed both auxin-like and cytokinin-like effects of synthetic compounds Ivin, Methyur, and Kamethur on the plant cell elongation, division, and differentiation, which are the main processes of organogenesis of shoots and roots of the miniature rose (R. mini L.) in vitro.
Analyzing the relationship between the chemical structure and biological activity of new synthetic compounds, derivatives of pyrimidine (No. 1, 4, 5, and 7), it can be assumed that the high auxin-like activity of these compounds is associated with the presence of substituents in their chemical structure: compound No. 1 contains an ethylthio group in position 2, a hydroxyl group in position 4 and a methyl group in position 6; compound No. 4 contains an isopropyl substituent in position 2, a hydroxyl group in position 4, and a methyl group in position 6; compound No. 5 is the sodium salt of 4-hydroxypyrimidine-2-thiolate; compound No. 7 contains a benzylthio group in position 2 and a hydroxyl group in position 4 (Table 1).
Table 1: Chemical structure, name, and relative molecular weight of auxins IAA and NAA, synthetic compounds Ivin, Methyur, Kamethur, and synthetic compounds, derivatives of pyrimidine (No. 1 - 7).
At the same time, a decrease in the auxin-like activity of new synthetic compounds, derivatives of pyrimidine (No. 2, 3, and 6) can be explained by the presence of substituents in the chemical structures of these compounds: compound No. 2 contains a propylthio group in position 2, a hydroxyl group in position 4 and a methyl group in position 6; compound No. 3 contains a benzylthio group in position 2, a hydroxyl group in position 4 and a methyl group in position 6; compound No. 6 contains a methylthio group in position 2 and a hydroxyl group in position 4 (Table 1).
It is possible to assume that the high growth regulatory activity of most active synthetic compounds Ivin, Methyur, Kamethur, and new synthetic compounds, derivatives of pyrimidine (No. 1, 4, 5 and 7), is explained by their specific auxin-like stimulating effect on the proliferation, elongation and differentiation of plant cells, which are the main processes of the formation and growth of plant roots, as well on the biosynthesis, metabolism and signaling of endogenous auxins in plant cells [40-44].
It is planned to further compare the results obtained in this work on the effect of synthetic compounds Ivin, Methyur, Kamethur and new synthetic compounds, derivatives of pyrimidine (No. 1 - 7) on the rooting of isolated stem cuttings of haricot bean (P. vulgaris L.) variety Bilozernaya with the regulating effect of all these synthetic compounds, used for seed treatment, on the growth and development of haricot bean plants (P. vulgaris L.) in the vegetative stage. These studies will contribute to the further development of new effective growth regulators used in agriculture to improve the growth and development and increase the yield of haricot bean plants (P. vulgaris L.).
The data obtained indicate the prospect of practical use of synthetic compounds Ivin, Methyur, Kamethur, and new synthetic compounds, derivatives of pyrimidine (No. 1, 4, 5 and 7) as effective substitutes for phytohormones auxins to improve the vegetative propagation of haricot bean plants (Phaseolus vulgaris L.) and other plant species of the family Fabaceae by stem cuttings.
- Tsygankova VA. Genetic Control and Phytohormonal Regulation of Plant Embryogenesis. Int. J. Med. Biotechnol. Genetics. 2015; 03(1): 9-20. DOI: 10.19070/2379-1020-150003.https://doi.org/10.19070/2379-1020-150003.
- Salaün C, Lepiniec L, Dubreucq B. Genetic and Molecular Control of Somatic Embryogenesis. Plants (Basel). 2021 Jul 17;10(7):1467. doi: 10.3390/plants10071467. PMID: 34371670; PMCID: PMC8309254.
- Su YH, Liu YB, Zhang XS. Auxin-cytokinin interaction regulates meristem development. Mol Plant. 2011 Jul;4(4):616-25. doi: 10.1093/mp/ssr007. Epub 2011 Feb 28. PMID: 21357646; PMCID: PMC3146736.
- Schaller GE, Bishopp A, Kieber JJ. The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell. 2015 Jan;27(1):44-63. doi: 10.1105/tpc.114.133595. Epub 2015 Jan 20. PMID: 25604447; PMCID: PMC4330578.
- Perianez-Rodriguez J, Manzano C, Moreno-Risueno MA. Post-embryonic organogenesis and plant regeneration from tissues: two sides of the same coin? Front Plant Sci. 2014 May 26;5:219. doi: 10.3389/fpls.2014.00219. PMID: 24904615; PMCID: PMC4033269.
- Pan R, Tian X. Comparative effect of IBA, BSSA and 5,6-Cl2-IAA-Me on the rooting of hypocotyl in mung bean. Plant Growth Regulation. 1999; 28: 91-98. DOI: https://doi.org/10.1023/A:1006154426941.
- Gaspar Th, Kevers C, Faivre-Rampant O, Crèvecoeur M, Penel Cl, Greppin H, Dommes J. Changing concepts in plant hormone action. In Vitro Cellular & Developmental Biology – Plant. 2003; 39(2): 85-106.
- George EF, Hall MA, De Klerk GJ. Plant Growth Regulators I: Introduction; Auxins, their Analogues and Inhibitors. Chapter 5. In: Plant Propagation by Tissue Culture. 3rd Ed. Springer. 2008; 175-204.
- George EF, Hall MA, De Klerk GJ. Plant Growth Regulators II: Cytokinins, their Analogues and Antagonists. Chapter 6. In: Plant Propagation by Tissue Culture. 3rd Ed. Springer. 2008; 205-226.
- Tadino VLA, Faez JM, Christiaens LE, Kevers C, Gaspar T, Dommes J. Synthesis and activity of another seleniated auxin: 2,4-dichlorophenylselenoacetic acid. Plant Growth Regulation. 2003; 40(3):197-200. DOI: 10.1023/A:1025016007105.
- Novickienė L, Asakavičiūtė R. Analogues of auxin modifying growth and development of some monocot and dicot plants. Acta Physiol Plant. 2006; 28(6): 509-515. https://doi.org/10.1007/s11738-006-0046-6.
- Langhansova L, Marsik P, Vanek T. Regulation of tissue differentiation by plant growth regulators on tTCLs of Panax ginseng adventitious roots. Industrial Crops and Products. 2012; 35(1):154-159. https://doi.org/10.1016/j.indcrop.2011.06.028.
- Sauer M, Robert S, Kleine-Vehn J. Auxin: simply complicated. J Exp Bot. 2013 Jun;64(9):2565-77. doi: 10.1093/jxb/ert139. Epub 2013 May 13. PMID: 23669571.
- Rigal A, Ma Q, Robert S. Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci. 2014 Jul 30;5:373. doi: 10.3389/fpls.2014.00373. PMID: 25126092; PMCID: PMC4115670.
- Naseem A, Mohammad F. Thidiazuron: From Urea Derivative to Plant Growth Regulator. Singapore: Springer. 2018; 491. https://lib.ugent.be/catalog/ebk01:4100000002892653.
- Vylíčilová H, Bryksová M, Matušková V, Doležal K, Plíhalová L, Strnad M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules. 2020 May 29;10(6):832. doi: 10.3390/biom10060832. PMID: 32485963; PMCID: PMC7356397.
- Jameson PE. Zeatin: The 60th anniversary of its identification. Plant Physiol. 2023 May 2;192(1):34-55. doi: 10.1093/plphys/kiad094. PMID: 36789623; PMCID: PMC10152681.
- Blythe EK, Sibley JL, Tilt KM, Ruter JM. Methods of Auxin Application in Cutting Propagation: A Review of 70 Years of Scientific Discovery and Commercial Practice. J. Environ. Hort. 2007; 25(3): 166-185.
- Nowakowska K, Pacholczak A. The effect of auxins on the rooting of cuttings in several species of Fabaceae. Annals of Warsaw University of Life Sciences – SGGW Horticulture and Landscape Architecture. 2015; 36: 13-20.
- Shekhawat MS, Manokari M. Impact of Auxins on Vegetative Propagation through Stem Cuttings of Couroupita guianensis Aubl.: A Conservation Approach. Scientifica (Cairo). 2016;2016:6587571. doi: 10.1155/2016/6587571. Epub 2016 Dec 19. PMID: 28083155; PMCID: PMC5204115.
- Faisal M, Ahmad N, Anis M, Alatar AA, Qahtan AA. Auxin-cytokinin synergism in vitro for producing genetically stable plants of Ruta graveolens using shoot tip meristems. Saudi J Biol Sci. 2018 Feb;25(2):273-277. doi: 10.1016/j.sjbs.2017.09.009. Epub 2017 Sep 27. PMID: 29472777; PMCID: PMC5816005.
- Tien LH, Chac LD, Oanh LTL, Ly PT, Sau HT. Effect of auxins (IAA, IBA and NAA) on clonal propagation of Solanum procumbens stem cuttings. Plant Cell Biotechnology and Molecular Biology. 2020; 21(55-56): 113-120.
- Tsygankova VA, Bayer OO, Andrusevich YaV, Galkin AP, Brovarets VS, Yemets AI, Blume YaB. Screening of five and six-membered nitrogen-containing heterocyclic compounds as new effective stimulants of Linum usitatissimum L. organogenesis in vitro. Int. J. Med. Biotechnol. Genetics. 2016; 02(1): 1-9. http://scidoc.org/specialissues/IJMBG/S2/IJMBG- 2379-1020-S2-001.pdf.
- Tsygankova V, Andrusevich Ya, Shtompel O, Romaniuk O, Yaikova M, Hurenko A, Solomyanny R, Abdurakhmanova E, Klyuchko S, Holovchenko O, Bondarenko O, Brovarets V. Application of Synthetic Low Molecular Weight Heterocyclic Compounds Derivatives of Pyrimidine, Pyrazole and Oxazole in Agricultural Biotechnology as a New Plant Growth Regulating Substances. Int J Med Biotechnol Genetics. 2017; 02(2): 10-32. DOI: dx.doi.org/10.19070/2379-1020-SI02002.
- Tsygankova V, Andrusevich Ya, Kopich V, Shtompel O, Veligina Y, Pilyo S, Kachaeva M, Kornienko A, Brovarets V. Use of Oxazole and Oxazolopyrimidine to Improve Oilseed Rape Growth. Scholars Bulletin. 2018; 4(3): 301-312. DOI: 10.21276/sb.2018.4.3.8.
- Mohilnikova IV, Tsygankova VA, Gurenko AO, Brovarets VS, Bilko NМ, Yemets АІ. Influence of pyrazole derivatives on plant growth and development in vivo and in vitro. Reports of the National Academy of Sciences of Ukraine. 2021; 6: 108-119. DOI: https://doi.org/10.15407/dopovidi2021.06.108.
- TsygankovaV A, Andrusevich YaV, Shtompel OI, Solomyanny RM, Hurenko AO, Frasinyuk MS, Mrug GP, Shablykin OV, Pilyo SG, Kornienko AM, Brovarets VS. New Auxin and Cytokinin Related Compounds Based on Synthetic Low Molecular Weight Heterocycles, Chapter 16, In: Aftab T. (Ed.) Auxins, Cytokinins and Gibberellins Signaling in Plants, Signaling and Communication in Plants, Springer Nature Switzerland AG. 2022; 353-377. DOI: https://doi.org/10.1007/978-3-031-05427-3_16.
- Kawarada A, Nakayama M, Ota Ya, Takeuchi S. Use of pyridine derivatives as plant growth regulators and plant growth regulating agents. Patent DE2349745A1. 25 April 1974. https://patents.google.com/patent/DE2349745A1/en.
- Mansfield DJ, Rieck H, Greul J, Coqueron PY, Desbordes P, Genix P, Grosjean-Cournoyer MC, Perez J, Villier A. Pyridine derivatives as fungicidal compounds, Patent US7754741B2, 13 July 2010. https://patents.google.com/patent/US7754741.
- Boussemghoune MA, Whittingham WG, Winn CL, Glithro H, Aspinall MB. Pyrimidine derivatives and their use as herbicides. Patent US20120053053 A1. 1 March 2012. https://patents.google.com/patent/US20120053053.
- Minn K, Dietrich H, Dittgen J, Feucht D, Häuser-Hahn I, Rosinger CH. Pyrimidine Derivatives and Their Use for Controlling Undesired Plant Growth, Patent USOO8445408B2, 21 May 2013. https://patentimages.storage.googleapis.com/d1/52/26/d05b90090de7ff/US8445408.pdf.
- Cansev A, Gülen H. Zengin MK, Ergin S, Cansev M. Use of Pyrimidines in Stimulation of Plant Growth and Development and Enhancement of Stress Tolerance. WIPO Patent WO 2014/129996A1. 28 August 2014. https://patents.google.com/patent/WO2014129996A1/en.
- Mohilnikova IV, Tsygankova VA, Solomyannyi RM, Brovarets VS, Bilko NМ, Yemets АІ. Screening of growth-stimulating activity of synthetic compounds - pyrimidine derivatives, Reports of the National Academy of Sciences of Ukraine. 2020; 10: 62-70. https://doi.org/10.15407/dopovidi2020.10.062.
- Tsygankova VA, Voloshchuk IV, Andrusevich YaV, Kopich VM, Pilyo SG, Klyuchko SV, Kachaeva MV, Brovarets VS. Pyrimidine derivatives as analogues of plant hormones for intensification of wheat growth during the vegetation period. Journal of Advances in Biology. 2022; 15: 1-10. DOI: https://doi.org/10.24297/jab.v15i.9237.
- Tsygankova VA, Voloshchuk IV, Klyuchko SV, Pilyo SG, Brovarets VS, Kovalenko OA. The effect of pyrimidine and pyridine derivatives on the growth and productivity of sorghum. International Journal of Botany Studies. 2022; 7(5): 19-31. https://www.botanyjournals.com/archives/2022/vol7/issue5/7-4-28.
- Tsygankova VA, Voloshchuk IV, Kopich VM, Pilyo SG, Klyuchko SV, Brovarets VS. Studying the effect of plant growth regulators Ivin, Methyur and Kamethur on growth and productivity of sunflower. Journal of Advances in Agriculture. 2023; 14: 17-24. DOI: https://doi.org/10.24297/jaa.v14i.9453.
- Tsygankova VA, Oliynyk OO, Kvasko OYu, Pilyo SG, Klyuchko SV, Brovarets VS. Effect of Plant Growth Regulators Ivin, Methyur and Kamethur on the Organogenesis of Miniature Rose (Rosa mini L.) In Vitro. Int J Med Biotechnol Genetics. 2022. 02(1): 1-8. http://scidoc.org/IJMBG-2379-1020-S1-02-001.php.
- Basu RN. Effect of non-auxin chemicals on translocation of auxins in cuttings of Phaseolus vulgaris (L.) (kidney beans). J. Exp. Bot. 1972; 23:357-365.
- Bang H, Zhou XK, van Epps HL, Mazumdar M. Statistical Methods in Molecular Biology. Series: Methods in molecular biology. New York: Humana press. 2010; 13(620): 636.
- Cleland RE. Auxin and Cell Elongation. In: Davies, P.J. (eds) Plant Hormones. Springer. Dordrecht. 2010; 204-220. https://doi.org/10.1007/978-1-4020-2686-7_10.
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49-64. doi: 10.1146/annurev-arplant-042809-112308. PMID: 20192736; PMCID: PMC3070418.
- Lavy M, Estelle M. Mechanisms of auxin signaling. Development. 2016 Sep 15;143(18):3226-9. doi: 10.1242/dev.131870. PMID: 27624827; PMCID: PMC5047657.
- Leyser O. Auxin Signaling. Plant Physiol. 2018 Jan;176(1):465-479. doi: 10.1104/pp.17.00765. Epub 2017 Aug 17. PMID: 28818861; PMCID: PMC5761761.
- Majda M, Robert S. The Role of Auxin in Cell Wall Expansion. Int J Mol Sci. 2018 Mar 22;19(4):951. doi: 10.3390/ijms19040951. PMID: 29565829; PMCID: PMC5979272.